These regulations describe the purpose of CITES, the criteria for listing in the appendices, and the requirements for importing or exporting protected animals or plants.
Subpart A. Introduction
§ 23.1 What are the purposes of these regulations and CITES?
§ 23.2 How do I decide if these regulations apply to my shipment or me?
§ 23.3 What other wildlife and plant regulations may apply?
§ 23.4 What are Appendices I, II, and III?
§ 23.5 How are the terms used in these regulations defined?
§ 23.6 What are the roles of the Management and Scientific Authorities?
§ 23.7 What office do I contact for CITES information?
§ 23.8 What are the information collection requirements?
§ 23.9 Incorporation by reference.
Subpart B. Prohibitions, Exemptions, and Requirements
§ 23.14 [Reserved by 72 FR 48448]
§ 23.16 What are the U.S. CITES requirements for urine, feces, and synthetically derived DNA?
§ 23.18 What CITES documents are required to export Appendix-I wildlife?
§ 23.19 What CITES documents are required to export Appendix-I plants?
§ 23.20 What CITES documents are required for international trade?
§ 23.21 What happens if a country enters a reservation for a species?
§ 23.22 What are the requirements for in-transit shipments?
§ 23.23 What information is required on U.S. and foreign CITES documents?
§ 23.24 What code is used to show the source of the specimen?
§ 23.25 What additional information is required on a non–Party CITES document?
§ 23.26 When is a U.S. or foreign CITES document valid?
§ 23.27 What CITES documents do I present at the port?
Subpart C. Application Procedures, Criteria, and Conditions
§ 23.32 How do I apply for a U.S. CITES document?
§ 23.33 How is the decision made to issue or deny a request for a U.S. CITES document?
§ 23.35 What are the requirements for an import permit?
§ 23.36 What are the requirements for an export permit?
§ 23.37 What are the requirements for a re-export certificate?
§ 23.38 What are the requirements for a certificate of origin?
§ 23.39 What are the requirements for an introduction-from-the-sea certificate?
§ 23.40 What are the requirements for a certificate for artificially propagated plants?
§ 23.41 What are the requirements for a bred-in-captivity certificate?
§ 23.42 What are the requirements for a plant hybrid?
§ 23.43 What are the requirements for a wildlife hybrid?
§ 23.44 What are the requirements to travel internationally with my personally owned live wildlife?
§ 23.45 What are the requirements for a pre-Convention specimen?
§ 23.48 What are the requirements for a registered scientific institution?
§ 23.49 What are the requirements for an exhibition traveling internationally?
§ 23.50 What are the requirements for a sample collection covered by an ATA carnet?
§ 23.51 What are the requirements for issuing a partially completed CITES document?
§ 23.53 What are the requirements for obtaining a retrospective CITES document?
§ 23.54 How long is a U.S. or foreign CITES document valid?
§ 23.55 How may I use a CITES specimen after import into the United States?
§ 23.56 What U.S. CITES document conditions do I need to follow?
Subpart D. Factors Considered in Making Certain Findings
§ 23.60 What factors are considered in making a legal acquisition finding?
§ 23.61 What factors are considered in making a non-detriment finding?
§ 23.62 What factors are considered in making a finding of not for primarily commercial purposes?
§ 23.63 What factors are considered in making a finding that an animal is bred in captivity?
§ 23.64 What factors are considered in making a finding that a plant is artificially propagated?
Subpart E. International Trade in Certain Specimens
§ 23.68 How can I trade internationally in roots of American ginseng?
§ 23.71 How can I trade internationally in sturgeon caviar?
§ 23.72 How can I trade internationally in plants?
§ 23.73 How can I trade internationally in timber?
§ 23.74 How can I trade internationally in personal sport-hunted trophies?
§ 23.75 How can I trade internationally in vicuña (Vicugna vicugna)?
Subpart F. Disposal of Confiscated Wildlife and Plants
§ 23.78 What happens to confiscated wildlife and plants?
§ 23.79 How may I participate in the Plant Rescue Center Program?
Subpart G. Cites Administration
§ 23.84 What are the roles of the Secretariat and the committees?
§ 23.85 What is a meeting of the Conference of the Parties (CoP)?
§ 23.86 How can I obtain information on a CoP?
§ 23.87 How does the United States develop documents and negotiating positions for a CoP?
§ 23.88 What are the resolutions and decisions of the CoP?
Subpart H. Lists of Species
§ 23.89 What are the criteria for listing species in Appendix I or II?
§ 23.90 What are the criteria for listing species in Appendix III?
§ 23.91 How do I find out if a species is listed?
§ 23.92 Are any wildlife or plants, and their parts, products, or derivatives, exempt?
Subpart A. Introduction
§ 23.1 What are the purposes of these regulations and CITES?
(a) Treaty. The regulations in this part implement the Convention on International Trade in Endangered Species of Wild Fauna and Flora, also known as CITES, the Convention, the Treaty, or the Washington Convention, TIAS (Treaties and Other International Acts Series) 8249.
(b) Purpose. The aim of CITES is to regulate international trade in wildlife and plants, including parts, products, and derivatives, to ensure it is legal and does not threaten the survival of species in the wild. Parties, recognize that:
(1) Wildlife and plants are an irreplaceable part of the natural systems of the earth and must be protected for this and future generations.
(2) The value of wildlife and plants is ever-growing from the viewpoints of aesthetics, science, culture, recreation, and economics.
(3) Although countries should be the best protectors of their own wildlife and plants, international cooperation is essential to protect wildlife and plant species from over-exploitation through international trade.
(4) It is urgent that countries take appropriate measures to prevent illegal trade and ensure that any use of wildlife and plants is sustainable.
(c) National legislation. We, the U.S. Fish and Wildlife Service (FWS), implement CITES through the Endangered Species Act (ESA).
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.2 How do I decide if these regulations apply to my shipment or me?
If you are engaging in activities with specimens of CITES–listed species these regulations apply to you.
Credits
[79 FR 30419, May 27, 2014]
§ 23.3 What other wildlife and plant regulations may apply?
(a) You may need to comply with other regulations in this subchapter that require a permit or have additional restrictions. Many CITES species are also covered by one or more parts of this subchapter or title and have additional requirements:
(1) Part 15 (exotic birds).
(2) Part 16 (injurious wildlife).
(3) Parts 17 of this subchapter and 222, 223, and 224 of this title (endangered and threatened species).
(4) Parts 18 of this subchapter and 216 of this title (marine mammals).
(5) Part 20 (migratory bird hunting).
(6) Part 21 (migratory birds).
(7) Part 22 (bald and golden eagles).
(b) If you are applying for a permit, you must comply with the general permit procedures in part 13 of this subchapter. Definitions and a list of birds protected under the Migratory Bird Treaty Act can be found in part 10 of this subchapter.
(c) If you are importing (including introduction from the sea), exporting, or re-exporting wildlife or plants, you must comply with the regulations in part 14 of this subchapter for wildlife or part 24 of this subchapter for plants. Activities with plants are also regulated by the U.S. Department of Agriculture, Animal and Plant Health Inspection Service (APHIS) and Department of Homeland Security, U.S. Customs and Border Protection (CBP), in 7 CFR parts 319, 355, and 356.
(d) You may also need to comply with other Federal, State, tribal, or local requirements.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.4 What are Appendices I, II, and III?
Species are listed by the Parties in one of three Appendices to the Treaty (see subpart H of this part), each of which provides a different level of protection and is subject to different requirements. Parties regulate trade in specimens of Appendix-I, -II, and -III species and their parts, products, and derivatives through a system of permits and certificates (CITES documents). Such documents enable Parties to monitor the effects of the volume and type of trade to ensure trade is legal and not detrimental to the survival of the species.
(a) Appendix I includes species threatened with extinction that are or may be affected by trade. Trade in Appendix-I specimens may take place only in exceptional circumstances.
(b) Appendix II includes species that are not presently threatened with extinction, but may become so if their trade is not regulated. It also includes species that need to be regulated so that trade in certain other Appendix-I or -II species may be effectively controlled; these species are most commonly listed due to their similarity of appearance to other related CITES species.
(c) Appendix III includes species listed unilaterally by a range country to obtain international cooperation in controlling trade.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.5 How are the terms used in these regulations defined?
In addition to the definitions contained in part 10 of this subchapter, and unless the context otherwise requires, in this part:
Affected by trade means that either a species is known to be in trade and the trade has or may have a detrimental impact on the status of the species, or a species is suspected to be in trade or there is demonstrable potential international demand for the species that may be detrimental to the survival of the species in the wild.
Annotation means an official footnote to the listing of a species in the CITES Appendices. A reference annotation provides information that further explains the listing (such as “p.e.” for possibly extinct). A substantive annotation is an integral part of a species listing. It designates whether the listing includes or excludes a geographically separate population, subspecies, species, group of species, or higher taxon, and the types of specimens included in or excluded from the listing, such as certain parts, products, or derivatives. A substantive annotation may designate export quotas adopted by the CoP. For species transferred from Appendix I to II subject to an annotation relating to specified types of specimens, other types of specimens that are not specifically included in the annotation are treated as if they are Appendix–I specimens.
Appropriate and acceptable destination, when used in an Appendix–II listing annotation for the export of, or international trade in, live animals, means that the Management Authority of the importing country has certified, based on advice from the Scientific Authority of that country, that the proposed recipient is suitably equipped to house and care for the animal (see criteria in § 23.65). Such certification must be provided before a CITES document is issued by the Management Authority of the exporting or re-exporting country.
Artificially propagated means a cultivated plant that meets the criteria in § 23.64.
ATA carnet means a type of international customs document (see § 23.50). ATA is a combination of the French and English words “Admission Temporaire/Temporary Admission.”
Bred for commercial purposes means any specimen of an Appendix–I wildlife species bred in captivity for commercial purposes. Any Appendix–I specimen that does not meet the definition of “bred for noncommercial purposes” is considered to be bred for commercial purposes.
Bred for noncommercial purposes means any specimen of an Appendix–I wildlife species bred in captivity for noncommercial purposes, where each donation, exchange, or loan of the specimen is noncommercial.
Bred in captivity means wildlife that is captive-bred and meets the criteria in § 23.63.
Captive-bred means wildlife that is the offspring (first (F1) or subsequent generations) of parents that either mated or otherwise transferred egg and sperm under controlled conditions if reproduction is sexual, or of a parent that was maintained under controlled conditions when development of the offspring began if reproduction is asexual, but does not meet the bred-in-captivity criteria (see § 23.63).
Certificate means a CITES document or CITES exemption document that identifies on its face the type of certificate it is, including re-export certificate, introduction-from-the-sea certificate, and certificate of origin.
CITES document or CITES exemption document means any certificate, permit, or other document issued by a Management Authority of a Party or a competent authority of a non–Party whose name and address is on file with the Secretariat to authorize the international movement of CITES specimens.
Commercial means related to an activity, including actual or intended import, export, re-export, sale, offer for sale, purchase, transfer, donation, exchange, or provision of a service, that is reasonably likely to result in economic use, gain, or benefit, including, but not limited to, profit (whether in cash or in kind).
Coral (dead) means pieces of stony coral that contain no living coral tissue and in which the structure of the corallites (skeletons of the individual polyps) is still intact and the specimens are therefore identifiable to the level of species or genus. See also § 23.23(c)(13).
Coral fragments, including coral gravel and coral rubble, means loose pieces of broken finger-like stony coral between 2 and 30 mm measured in all directions that contain no living coral tissue and are not identifiable to the level of genus (see § 23.92for exemptions).
Coral (live) means pieces of stony coral that are alive and are therefore identifiable to the level of species or genus. See also § 23.23(c)(13).
Coral rock means hard consolidated material greater than 30 mm measured in any direction that consists of pieces of stony coral that contain no living coral tissue and possibly also cemented sand, coralline algae, or other sedimentary rocks. Coral rock includes live rock and substrate, which are terms for pieces of coral rock to which are attached live specimens of other invertebrate species or coralline algae that are not listed in the CITES Appendices. See also § 23.23(c)(13).
Coral sand means material that consists entirely or in part of finely crushed stony coral no larger than 2 mm measured in all directions that contains no living coral tissue and is not identifiable to the level of genus (see § 23.92 for exemptions).
Coral (stony) means any coral in the orders Helioporacea, Milleporina, Scleractinia, Stolonifera, and Stylasterina.
Country of origin means the country where the wildlife or plant was taken from the wild or was born or propagated in a controlled environment, except in the case of a plant specimen that qualified for an exemption under the provisions of CITES, the country of origin is the country in which the specimen ceased to qualify for the exemption.
Cultivar means a horticulturally derived plant variety that: has been selected for a particular character or combination of characters; is distinct, uniform, and stable in these characters; and when propagated by appropriate means, retains these characters. The cultivar name and description must be formally published in order to be recognized under CITES.
Cultivated means a plant grown or tended by humans for human use. A cultivated plant can be treated as artificially propagated under CITES only if it meets the criteria in § 23.64.
Export means to send, ship, or carry a specimen out of a country (for export from the United States, see part 14 of this subchapter).
Flasked means plant material obtained in vitro, in solid or liquid media, transported in sterile containers.
Household effect means a dead wildlife or plant specimen that is part of a household move and meets the criteria in § 23.15.
Hybrid means any wildlife or plant that results from a cross of genetic material between two separate taxa when one or both are listed in Appendix I, II, or III. See § 23.42 for plant hybrids and § 23.43 for wildlife hybrids.
Import means to bring, ship, or carry a specimen into a country (for import into the United States, see part 14 of this subchapter).
International trade means the import, introduction from the sea, export, or re-export across jurisdictional or international boundaries for any purpose whether commercial or noncommercial.
In-transit shipment means the transshipment of any wildlife or plant through an intermediary country when the specimen remains under customs control and either the shipment meets the requirements of § 23.22 or the sample collection covered by an ATA carnet meets the requirements of § 23.50.
Introduction from the sea means transportation into a country of specimens of any species that were taken in the marine environment not under the jurisdiction of any country, i.e., taken in those marine areas beyond the areas subject to the sovereignty or sovereign rights of a country consistent with international law, as reflected in the United Nations Convention on the Law of the Sea.
ISO country code means the two-letter country code developed by the International Organization for Standardization (ISO) to represent the name of a country and its subdivisions.
Live rock see the definition for coral rock.
Management Authority means a governmental agency officially designated by, and under the supervision of, either a Party to implement CITES, or a non–Party to serve in the role of a Management Authority, including the issuance of CITES documents on behalf of that country.
Noncommercial means related to an activity that is not commercial. Noncommercial includes, but is not limited to, personal use.
Non–Party means a country that has not deposited an instrument of ratification, acceptance, approval, or accession to CITES with the Depositary Government (Switzerland), or a country that was a Party but subsequently notified the Depositary Government of its denunciation of CITES and the denunciation is in effect.
Offspring of first generation (F1) means a wildlife specimen produced in a controlled environment from parents at least one of which was conceived in or taken from the wild.
Offspring of second generation (F2) or subsequent generations means a wildlife specimen produced in a controlled environment from parents that were also produced in a controlled environment.
Parental stock means the original breeding or propagating specimens that produced the subsequent generations of captive or cultivated specimens.
Party means a country that has given its consent to be bound by the provisions of CITES by depositing an instrument of ratification, acceptance, approval, or accession with the Depositary Government (Switzerland), and for which such consent is in effect.
Permit means a CITES document that identifies on its face import permit or export permit.
Personal effect means a dead wildlife or plant specimen, including a tourist souvenir, that is worn as clothing or accessories or is contained in accompanying baggage and meets the criteria in § 23.15.
Personal use means use that is not commercial and is for an individual's own consumption or enjoyment.
Precautionary measures means the actions taken that will be in the best interest of the conservation of the species when there is uncertainty about the status of a species or the impact of trade on the conservation of a species.
Pre–Convention means a specimen that was acquired (removed from the wild or born or propagated in a controlled environment) before the date the provisions of the Convention first applied to the species and that meets the criteria in § 23.45, and any product (including a manufactured item) or derivative made from such specimen.
Primarily commercial purposes means an activity whose noncommercial aspects do not clearly predominate (see § 23.62).
Propagule means a structure, such as a cutting, seed, or spore, which is capable of propagating a plant.
Ranched wildlife means specimens of animals reared in a controlled environment that were taken from the wild as eggs or juveniles where they would otherwise have had a very low probability of surviving to adulthood. See also § 23.34.
Readily recognizable means any specimen that appears from a visual, physical, scientific, or forensic examination or test; an accompanying document, packaging, mark, or label; or any other circumstances to be a part, product, or derivative of any CITES wildlife or plant, unless such part, product, or derivative is specifically exempt from the provisions of CITES or this part.
Re-export means to send, ship, or carry out of a country any specimen previously imported into that country, whether or not the specimen has been altered since import.
Reservation means the action taken by a Party to inform the Secretariat that it is not bound by the effect of a specific listing (see § 23.21).
Scientific Authority means a governmental or independent scientific institution or entity officially designated by either a Party to implement CITES, or a non–Party to serve the role of a Scientific Authority, including making scientific findings.
Secretariat means the entity designated by the Treaty to perform certain administrative functions (see § 23.84).
Shipment means any CITES specimen in international trade whether for commercial or noncommercial use, including any personal item.
Species means any species, subspecies, hybrid, variety, cultivar, color or morphological variant, or geographically separate population of that species.
Specimen means any wildlife or plant, whether live or dead. This term includes any readily recognizable part, product, or derivative unless otherwise annotated in the Appendices.
Sustainable use means the use of a species in a manner and at a level that maintains wild populations at biologically viable levels for the long term. Such use involves a determination of the productive capacity of the species and its ecosystem to ensure that utilization does not exceed those capacities or the ability of the population to reproduce, maintain itself, and perform its role or function in its ecosystem.
Trade means the same as international trade.
Transit see the definition for in-transit shipment.
Traveling exhibition means a display of live or dead wildlife or plants for entertainment, educational, cultural, or other display purposes that is temporarily moving internationally.
Credits
[79 FR 30419, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.6 What are the roles of the Management and Scientific Authorities?
Under Article IX of the Treaty, each Party must designate a Management and Scientific Authority to implement CITES for that country. If a non–Party wants to trade with a Party, it must also designate such Authorities. The names and addresses of these offices must be sent to the Secretariat to be included in the Directory. In the United States, different offices within the FWS have been designated the Scientific Authority and Management Authority, which for purposes of this section includes FWS Law Enforcement. When offices share activities, the Management Authority is responsible for dealing primarily with management and regulatory issues and the Scientific Authority is responsible for dealing primarily with scientific issues. The offices do the following:
|
Roles
|
U.S. Scientific Authority
|
U.S. Management Authority
|
|
(a) Provide scientific advice and recommendations, including advice on biological findings for applications for certain CITES documents, registrations, and export program approvals. Evaluate the conservation status of species to determine if a species listing or change in a listing is warranted. Interpret listings and review nomenclatural issues.
|
x
|
|
|
(b) Review applications for CITES documents and issue or deny them based on findings required by CITES.
|
x
|
|
|
(c) Communicate with the Secretariat and other countries on scientific, administrative, and enforcement issues.
|
x
|
x
|
|
(d) Ensure that export of Appendix-II specimens is at a level that maintains a species throughout its range at a level consistent with its role in the ecosystems in which it occurs and well above the level at which it might become eligible for inclusion in Appendix I.
|
x
|
|
|
(e) Monitor trade in all CITES species and produce annual reports on CITES trade.
|
x
|
|
|
(f) Collect the cancelled foreign export permit or re-export certificate and any corresponding import permit presented for import of any CITES specimen. Collect a copy of the validated U.S. export permit or re-export certificate presented for export or re-export of any CITES specimen.
|
x
|
|
|
(g) Produce biennial reports on legislative, regulatory, and administrative measures taken by the United States to enforce the provisions of CITES.
|
x
|
|
|
(h) Coordinate with State and tribal governments and other Federal agencies on CITES issues, such as the status of native species, development of policies, negotiating positions, and law enforcement activities.
|
x
|
x
|
|
(i) Communicate with the scientific community, the public, and media about CITES issues. Conduct public meetings and publish notices to gather input from the public on the administration of CITES and the conservation and trade status of domestic and foreign species traded internationally.
|
x
|
x
|
|
(j) Represent the United States at the meetings of the CoP, on committees (see subpart G of this part), and on CITES working groups. Consult with other countries on CITES issues and the conservation status of species. Prepare discussion papers and proposals for new or amended resolutions and species listings for consideration at the CoP.
|
x
|
x
|
|
(k) Provide assistance to APHIS and CBP for the enforcement of CITES. Cooperate with enforcement officials to facilitate the exchange of information between enforcement bodies and for training purposes.
|
x
|
x
|
|
(l) Provide financial and technical assistance to other governmental agencies and CITES officials of other countries.
|
x
|
x
|
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.7 What office do I contact for CITES information?
Contact the following offices to receive information about CITES:
|
Type of information |
Office to contact |
||
|
(a) CITES administrative and management issues: |
|||
|
(1) CITES documents, including application forms and procedures; lists of registered scientific institutions and operations breeding Appendix-I wildlife for commercial purposes; and reservations |
U.S. Management Authority, U.S. Fish and Wildlife Service Headquarters, MS: IA, 5275 Leesburg Pike, Falls Church, VA 22041-3803, Toll Free: (800) 358-2104/permit questions, Tel: (703) 358-2095/other questions, Fax: (703) 358-2281/permits, Fax: (703) 358-2298/other issues, Email: managementauthority@fws.gov, Web site: http://www.fws.gov/international and http://www.fws.gov/permits. |
||
|
(2) Information on the CoP |
|||
|
(3) List of CITES species |
|||
|
(4) Names and addresses of other countries' Management and Scientific Authority offices |
|||
|
(5) Notifications, resolutions, and decisions |
|||
|
(6) Standing Committee documents and issues |
|||
|
(7) State and tribal export programs |
|||
|
(b) Scientific issues: |
|||
|
(1) Animals and Plants Committees documents and issues |
U.S. Scientific Authority, U.S. Fish and Wildlife Service Headquarters, MS: IA, 5275 Leesburg Pike, Falls Church, VA 22041-3803, Tel: (703) 358-1708, Fax: (703) 358-2276, Email: scientificauthority@fws.gov, Web site: http://www.fws.gov/international. |
||
|
(2) Findings of non-detriment and suitability of facilities, and other scientific findings |
|||
|
(3) Listing of species in the Appendices and relevant resolutions |
|||
|
(4) Names and addresses of other countries' Scientific Authority offices and scientists involved with CITES-related issues |
|||
|
(5) Nomenclatural issues |
|||
|
(c) Wildlife clearance procedures: |
|||
|
(1) CITES replacement tags |
Law Enforcement, U.S. Fish and Wildlife Service Headquarters, MS: OLE, 5275 Leesburg Pike, Falls Church, VA 22041-3803, Tel: (703) 358-1949, Fax: (703) 358-2271, Web site: http://www.fws.gov/le. |
||
|
(2) Information about wildlife port office locations |
|||
|
(3) Information bulletins |
|||
|
(4) Inspection and clearance of wildlife shipments involving import, introduction from the sea, export, and re-export, and filing a Declaration of Importation or Exportation of Fish or Wildlife (Form 3-177) |
|||
|
(5) Validation, certification, or cancellation of CITES wildlife documents |
|||
|
(d) APHIS plant clearance procedures: |
U.S. Department of Agriculture APHIS/PPQ |
||
|
(1) Information about plant port office locations |
4700 River Road |
||
|
(2) Inspection and clearance of plant shipments involving: |
Riverdale, Maryland 20737-1236 |
||
|
(i) Import and introduction from the sea of living plants |
Toll Free: (877) 770-5990/permit questions |
||
|
(ii) Export and re-export of living and nonliving plants |
Tel: (301) 734-8891/other CITES issues |
||
|
(3) Validation or cancellation of CITES plant documents for the type of shipments listed in paragraph (d)(2) of this section |
Fax: (301) 734-5786/permit questions |
||
|
Fax: (301) 734–5276/other CITES issues |
|||
|
Website: http://www.aphis.usda.gov/plant_health |
|||
|
(e) CBP plant clearance procedures: |
Department of Homeland Security |
||
|
(1) Inspection and clearance of plant shipments involving: |
U.S. Customs and Border Protection |
||
|
(i) Import and introduction from the sea of nonliving plants |
Office of Field Operations |
||
|
(ii) Import of living plants from Canada at designated border ports (7 CFR 319.37-14(b) and 50 CFR 24.12(d)) |
Agriculture Programs and Liaison |
||
|
(2) Cancellation of CITES plant documents for the type of shipments listed in paragraph (e)(1) of this section |
1300 Pennsylvania Avenue, NW, Room 2.5 B |
||
|
Washington, DC 20229 |
|||
|
Tel: (202) 344–3298 |
|||
|
Fax: (202) 344–1442 |
|||
|
(f) General information on CITES: |
CITES Secretariat |
||
|
(1) CITES export quota information |
Website: http://www.cites.org |
||
|
(2) CITES' Guidelines for transport and preparation for shipment of live wild animals and plants |
|||
|
(3) Information about the Secretariat |
|||
|
(4) Names and addresses of other countries' Management and Scientific Authority offices |
|||
|
(5) Official documents, including resolutions, decisions, notifications, CoP documents, and committee documents |
|||
|
(6) Official list of CITES species and species database |
|||
|
(7) Text of the Convention |
|||
Credits
[79 FR 30420, May 27, 2014; 79 FR 43966, July 29, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
§ 23.8 What are the information collection requirements?
The Office of Management and Budget approved the information collection requirements for application forms and reports contained in this part and assigned OMB Control Number 1018–0093. We cannot collect or sponsor a collection of information and you are not required to provide information unless it displays a currently valid OMB control number.
Credits
[79 FR 30420, May 27, 2014]
§ 23.9 Incorporation by reference.
(a) Certain material is incorporated by reference into this part with the approval of the Director of the Federal Register in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. You may inspect copies at the U.S. Management Authority, U.S. Fish and Wildlife Service Headquarters, MS. IA, 5275 Leesburg Pike, Falls Church, VA 22041–3803, or at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202–741–6030, or go to: http://www.archives.gov/federal_register/code_of_federal_regulations/ibr_locations.html.
(b) International Air Transport Association (IATA), 800 Place Victoria, P.O. Box 113, Montreal, Quebec, Canada H4Z 1M1, 1–800–716–6326, http://www.iata.org.
(1) Live Animals Regulations (LAR) 40th edition, effective October 1, 2013, into §§ 23.23, 23.26, and 23.56.
(2) Perishable Cargo Regulations (PCR) 13th edition, effective July 1, 2013, into §§ 23.23, 23.26, and 23.56.
Credits
[79 FR 30420, May 27, 2014; 79 FR 43967, July 29, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
Subpart B. Prohibitions, Exemptions, and Requirements
Except as provided in § 23.92, it is unlawful for any person subject to the jurisdiction of the United States to conduct any of the following activities unless they meet the requirements of this part:
(a) Import, export, re-export, or engage in international trade with any specimen of a species listed in Appendix I, II, or III of CITES.
(b) Introduce from the sea any specimen of a species listed in Appendix I or II of CITES.
(c) Possess any specimen of a species listed in Appendix I, II, or III of CITES imported, exported, re-exported, introduced from the sea, or traded contrary to the provisions of CITES, the ESA, or this part.
(d) Use any specimen of a species listed in Appendix I, II, or III of CITES for any purpose contrary to what is allowed under § 23.55.
(e) Violate any other provisions of this part.
(f) Attempt to commit, solicit another to commit, or cause to be committed any of the activities described in paragraphs (a) through (e) of this section.
Credits
[79 FR 30420, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.14 [Reserved by 72 FR 48448]
§ 23.15 How may I travel internationally with my personal or household effects, including tourist souvenirs?
(a) Purpose. Article VII(3) of the Treaty recognizes a limited exemption for the international movement of personal and household effects.
(b) Stricter national measures. The exemption for personal and household effects does not apply if a country prohibits or restricts the import, export, or re-export of the item.
(1) You or your shipment must be accompanied by any document required by a country under its stricter national measures.
(2) In the United States, you must obtain any permission needed under other regulations in this subchapter (see § 23.3).
(c) Required CITES documents. You must obtain a CITES document for personal or household effects and meet the requirements of this part if one of the following applies:
(1) The Management Authority of the importing, exporting, or re-exporting country requires a CITES document.
(2) You or your shipment does not meet all of the conditions for an exemption as provided in paragraphs (d) through (f) of this section.
(3) The personal or household effect for the following species exceeds the quantity indicated in paragraphs (c)(3)(i) through (vi) in the table below:
|
Major group
|
Species (Appendix II only)
|
Type of specimen
|
Quantity [FN1]
|
|
Fishes
|
(i) Acipenseriformes (sturgeon, including paddlefish)
|
Sturgeon caviar (see § 23.71)
|
125 gm
|
|
Fishes
|
(ii) Hippocampus spp. (seahorses)
|
Dead specimens, parts, products (including manufactured items), and derivatives
|
4
|
|
Reptiles
|
(iii) Crocodylia (alligators, caimans, crocodiles, gavial)
|
Dead specimens, parts, products (including manufactured items), and derivatives
|
4
|
|
Molluscs
|
(iv) Strombus gigas (queen conch)
|
Shells
|
3
|
|
Molluscs
|
(v) Tridacnidae (giant clams)
|
Shells, each of which may be one intact shell or two matching halves
|
3 shells, total not exceeding 3 kg
|
|
Plants
|
(vi) Cactaceae (cacti)
|
Rainsticks
|
3
|
[FN1] To import, export, or re-export more than the quantity listed in the table, you must have a valid CITES document for the entire quantity.
(d) Personal effects. You do not need a CITES document to import, export, or re-export any legally acquired specimen of a CITES species to or from the United States if all of the following conditions are met:
(1) No live wildlife or plant (including eggs or non-exempt seeds) is included.
(2) No specimen from an Appendix–I species is included, except for certain worked African elephant ivory as provided in paragraph (f) of this section.
(3) The specimen and quantity of specimens are reasonably necessary or appropriate for the nature of your trip or stay and, if the type of specimen is one listed in paragraph (c)(3) of this section, the quantity does not exceed the quantity given in the table.
(4) You own and possess the specimen for personal use, including any specimen intended as a personal gift.
(5) You are either wearing the specimen as clothing or an accessory or taking it as part of your personal baggage, which is being carried by you or checked as baggage on the same plane, boat, vehicle, or train as you.
(6) The specimen was not mailed or shipped separately.
(e) Household effects. You do not need a CITES document to import, export, or re-export any legally acquired specimen of a CITES species that is part of a shipment of your household effects when moving your residence to or from the United States, if all of the following conditions are met:
(1) The provisions of paragraphs (d)(1) through (3) of this section are met.
(2) You own the specimen and are moving it for personal use.
(3) You import or export your household effects within 1 year of changing your residence from one country to another.
(4) The shipment, or shipments if you cannot move all of your household effects at one time, contains only specimens purchased, inherited, or otherwise acquired before you changed your residence.
(f) African elephant worked ivory. You may export or re-export from the United States worked African elephant (Loxodonta africana) ivory and then re-import it without a CITES document if all of the following conditions are met:
(1) The worked ivory is a personal or household effect that meets the requirements of paragraphs (c) through (e) of this section and you are a U.S. resident who owned the worked ivory before leaving the United States and intend to bring the item back to the United States.
(2) The ivory is pre–Convention (see § 23.45). (The African elephant was first listed in CITES on February 26, 1976.)
(3) You may not sell or transfer the ivory while outside the United States.
(4) The ivory is substantially worked and is not raw. Raw ivory means an African elephant tusk, or any piece of tusk, the surface of which, polished or unpolished, is unaltered or minimally carved, including ivory mounted on a stand or part of a trophy.
(5) When you return, you are able to provide records, receipts, or other documents to show that the ivory is pre–Convention and that you owned and registered it before you left the United States. To register such an item you must obtain one of the following documents:
(i) U.S. CITES pre–Convention certificate.
(ii) FWS Declaration of Importation or Exportation of Fish or Wildlife (Form 3–177).
(iii) Customs and Border Protection Certificate of Registration for Personal Effects Taken Abroad (Form 4457).
Credits
[73 FR 40986, July 17, 2008]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.16 What are the U.S. CITES requirements for urine, feces, and synthetically derived DNA?
(a) CITES documents. We do not require CITES documents to trade in urine, feces, or synthetically derived DNA.
(1) You must obtain any collection permit and CITES document required by the foreign country.
(2) If the foreign country requires you to have a U.S. CITES document for these kinds of samples, you must apply for a CITES document and meet the requirements of this part.
(b) Urine and feces. Except as provided in paragraph (a) of this section, we consider urine and feces to be wildlife byproducts, rather than parts, products, or derivatives, and exempt them from the requirements of CITES and this part.
(c) DNA. We differentiate between DNA directly extracted from blood and tissue and DNA synthetically derived as follows:
(1) A DNA sample directly derived from wildlife or plant tissue is regulated by CITES and this part.
(2) A DNA sample synthetically derived that does not contain any part of the original template is exempt from the requirements of CITES and this part.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.17 What are the requirements for CITES specimens traded internationally by diplomatic, consular, military, and other persons exempt from customs duties or inspections?
A specimen of a CITES species imported, introduced from the sea, exported, or re-exported by a person receiving duty-free or inspection exemption privileges under customs laws must meet the requirements of CITES and the regulations in this part.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.18 What CITES documents are required to export Appendix-I wildlife?
Answer the questions in the following decision tree to find the section in this part that applies to the type of CITES document you need to export Appendix-I wildlife. See § 23.20(d) for CITES exemption documents or § 23.92 for specimens that are exempt from the requirements of CITES and do not need CITES documents.
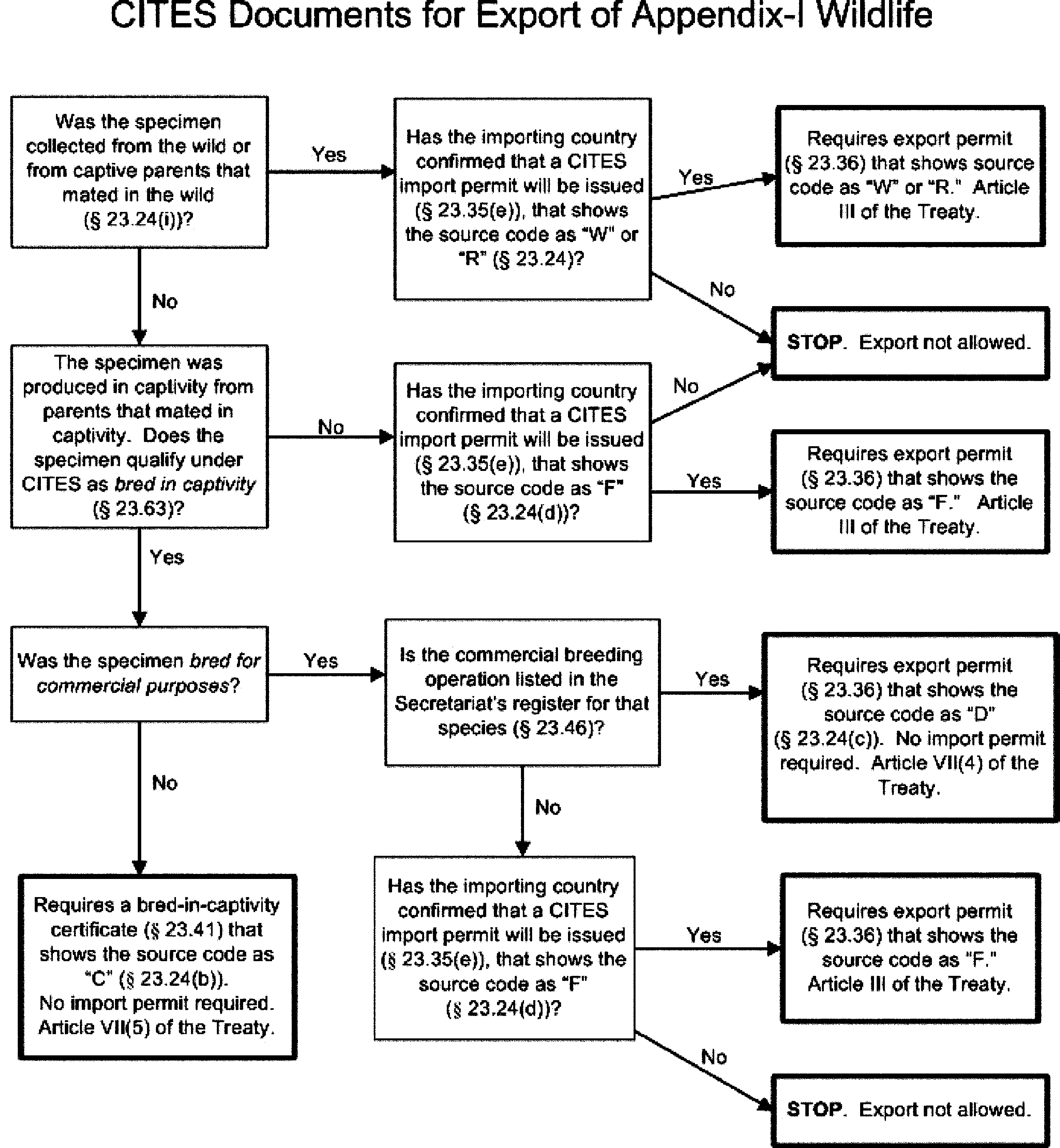
[79 FR 30420, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
§ 23.19 What CITES documents are required to export Appendix-I plants?
Answer the questions in the following decision tree to find the section in this part that applies to the type of CITES document you need to export Appendix–I plants. See § 23.20(d) for CITES exemption documents or § 23.92 for specimens that are exempt from the requirements of CITES and do not need CITES documents
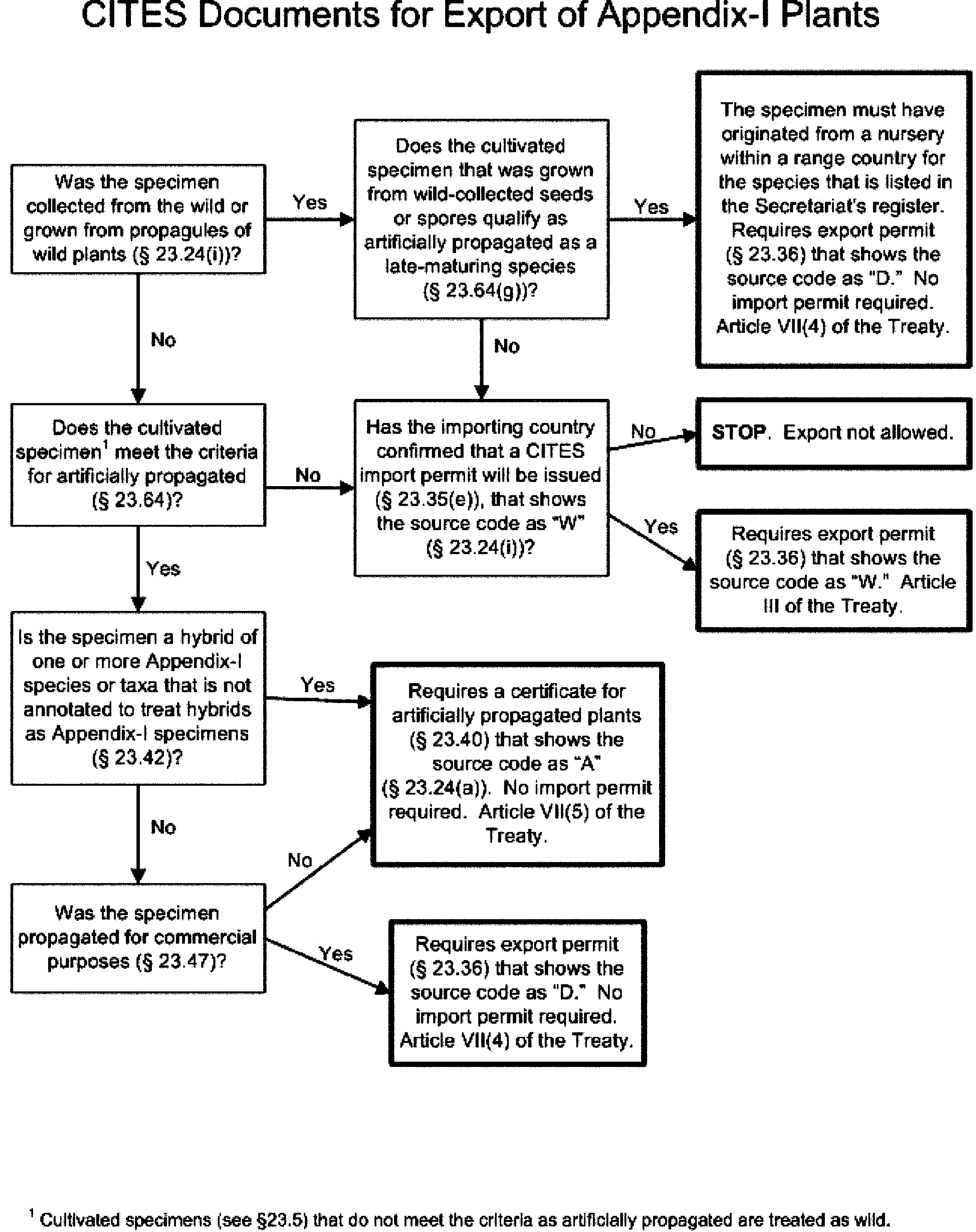
Credits
[79 FR 30421, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
§ 23.20 What CITES documents are required for international trade?
(a) Purpose. Articles III, IV, and V of the Treaty give the types of standard CITES documents that must accompany an Appendix–I, –II, or –III specimen in international trade. Articles VII and XIV recognize some exemptions and provide that a CITES document must accompany most exempt specimens.
(b) Stricter national measures. Before importing, introducing from the sea, exporting, or re-exporting a specimen, check with the Management Authorities of all countries concerned to obtain any documentation required under stricter national measures.
(c) CITES documents. Except as provided in the regulations in this part, you must have a valid CITES document to engage in international trade in any CITES specimen.
(d) CITES exemption documents. The following table lists the CITES exemption document that you must obtain before conducting a proposed activity with an exempt specimen (other than specimens exempted under § 23.92). If one of the exemptions does not apply to the specimen, you must obtain a CITES document as provided in paragraph (e) of this section. The first column in the following table alphabetically lists the type of specimen or activity that may qualify for a CITES exemption document. The last column indicates the section of this part that contains information on the application procedures, provisions, criteria, and conditions specific to each CITES exemption document, as follows:
|
Type of specimen or activity
|
Appendix
|
CITES exemption document
|
Section
|
|
(1) Artificially propagated plant (see paragraph (d)(4) of this section for an Appendix-I plant propagated for commercial purposes)
|
I, II, or III
|
CITES document with source code “A” [FN1]
|
23.40
|
|
(2) Artificially propagated plant from a country that has provided copies of the certificates, stamps, and seals to the Secretariat
|
II or III
|
Phytosanitary certificate with CITES statement [FN1]
|
23.23(f)
|
|
(3) Bred-in-captivity wildlife (see paragraph (d)(5) of this section for Appendix-I wildlife bred in captivity for commercial purposes)
|
I, II, or III
|
CITES document with source code “C” [FN1]
|
23.41
|
|
(4) Commercially propagated Appendix-I plant
|
I
|
CITES document with source code “D” [FN1]
|
23.47
|
|
(5) Commercially bred Appendix-I wildlife from a breeding operation registered with the CITES Secretariat
|
I
|
CITES document with source code “D” [FN1]
|
23.46
|
|
(6) Export of certain marine specimens protected under a pre-existing treaty, convention, or international agreement for that species
|
II
|
CITES document indicating that the specimen was taken in accordance with provisions of the applicable treaty, convention, or international agreement
|
23.36(e)
|
|
23.39(e)
|
|||
|
(7) Hybrid plants
|
I, II, or III
|
CITES document unless the specimen qualifies as an exempt plant hybrid
|
23.42
|
|
(8) Hybrid wildlife
|
I, II, or III
|
CITES document unless the specimen qualifies as an exempt wildlife hybrid
|
23.43
|
|
(9) In-transit shipment (see paragraph (d)(14) of this section for sample collections covered by an ATA carnet)
|
I, II, or III
|
CITES document designating importer and country of final destination
|
23.22
|
|
(10) Introduction from the sea under a pre-existing treaty, convention, or international agreement for that species
|
II
|
Document required by applicable treaty, convention, or international agreement, if appropriate
|
23.39(d)
|
|
(11) Noncommercial loan, donation, or exchange of specimens between scientific institutions registered with the CITES Secretariat
|
I, II, or III
|
A label indicating CITES and the registration codes of both institutions and, in the United States, a CITES certificate of scientific exchange that registers the institution [FN3]
|
23.48
|
|
(12) Personally owned live wildlife for multiple cross-border movements
|
I, II, or III
|
CITES certificate of ownership [FN2]
|
23.44
|
|
(13) Pre-Convention specimen
|
I, II, or III
|
CITES document indicating pre-Convention status [FN1]
|
23.45
|
|
(14) Sample collection covered by an ATA carnet
|
I [FN4] , II, or III
|
CITES document indicating sample collection [FN2]
|
23.50
|
|
(15) Traveling exhibition
|
I, II, or III
|
CITES document indicating specimens qualify as pre-Convention, bred in captivity, or artificially propagated [FN2]
|
23.49
|
[FN1] Issued by the Management Authority in the exporting or re-exporting country.
[FN2] Issued by the Management Authority in the owner's country of usual residence.
[FN3] Registration codes assigned by the Management Authorities in both exporting and importing countries.
[FN4] Appendix-I species bred in captivity or artificially propagated for commercial purposes (see §§ 23.46 and 23.47).
|
Appendix
|
Type of CITES document(s) required
|
|
I
|
Import permit (§ 23.35) and either an export permit (§ 23.36) or re-export certificate (§ 23.37)
|
|
II
|
Export permit (§ 23.36) or re-export certificate (§ 23.37)
|
|
III
|
Export permit (§ 23.36) if the specimen originated in a country that listed the species; certificate of origin (§ 23.38) if the specimen originated in a country other than the listing country
|
(f) Introduction-from-the-sea certificates. For introduction from the sea of Appendix–I or Appendix–II specimens, you must obtain an introduction-from-the-sea certificate before conducting the proposed activity, unless the exemption in paragraph (d)(10) of this section applies (see § 23.39). The export of a specimen that was previously introduced from the sea will be treated as an export (see § 23.36 for export, § 23.36(e) and § 23.39(e) for export of exempt specimens, or § 23.37 for re-export). Although an Appendix–III specimen does not require a CITES document to be introduced from the sea, the subsequent international trade of the specimen would be considered an export. For export of an Appendix–III specimen that was introduced from the sea you must obtain an export permit (§ 23.36) if the export is from the country that listed the species in Appendix III, a certificate of origin (§ 23.38) if the export is from a country other than the listing country, or a re-export certificate for all re-exports (§ 23.37).
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.21 What happens if a country enters a reservation for a species?
(a) Purpose. CITES is not subject to general reservations. Articles XV, XVI, and XXIII of the Treaty allow a Party to enter a specific reservation on a species listed in Appendix I, II, or III, or on parts, products, or derivatives of a species listed in Appendix III.
(b) General provision. A Party can enter a reservation in one of the following ways:
(1) A Party must provide written notification to the Depositary Government (Switzerland) on a specific new or amended listing in the Appendices within 90 days after the CoP that adopted the listing, or at any time for Appendix–III species.
(2) A country must provide written notification on a specific species listing when the country ratifies, accepts, approves, or accedes to CITES.
(c) Requesting the United States take a reservation. You may submit information relevant to the issue of whether the United States should take a reservation on a species listing to the U.S. Management Authority. The request must be submitted within 30 calendar days after the last day of the CoP where a new or amended listing of a species in Appendix I or II occurs, or at any time for a species (or its parts, products, or derivatives) listed in Appendix III.
|
If
|
Then
|
|
(1) The shipment is between a Party and a reserving Party, or the shipment is from a non-Party to a reserving Party and is in transit through a Party
|
The shipment must be accompanied by a valid CITES document(s) (see § 23.26) that indicates the CITES Appendix in which the species is listed.
|
|
(2) The shipment is from a reserving Party to another reserving Party [FN1] or non-Party and is in transit through a Party
|
The shipment must be accompanied by a valid CITES document(s) (see § 23.26) that indicates the CITES Appendix in which the species is listed. [FN2]
|
|
(3) The shipment is between a reserving Party and another reserving Party [FN1] or non-Party and is not in transit through a Party
|
No CITES document is required. [FN2]
|
[FN1] Both reserving Parties must have a reservation for the same species, and if the species is listed in Appendix III, a reservation for the same parts, products, and derivatives.
[FN2] CITES recommends that reserving Parties treat Appendix-I species as if listed in Appendix II and issue CITES documents based on Appendix-II permit criteria (see § 23.36). However, the CITES document must show the specimen as listed in Appendix I. If the United States entered a reservation, such a CITES document would be required.
(e) Reservations taken by countries. You may consult the CITES website or contact us (see § 23.7) for a list of countries that have taken reservations and the species involved.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.22 What are the requirements for in-transit shipments?
(a) Purpose. Article VII(1) of the Treaty allows for a shipment to transit an intermediary country that is a Party before reaching its final destination without the need for the intermediary Party to issue CITES documents. To control any illegal trade, Parties are to inspect, to the extent possible under their national legislation, specimens in transit through their territory to verify the presence of valid documentation. See § 23.50 for in-transit shipment of sample collections covered by an ATA carnet.
(b) Document requirements. An in-transit shipment does not require a CITES document from an intermediary country, but must be accompanied by all of the following documents:
(1) Unless the specimen qualifies for an exemption under § 23.92, a valid original CITES document, or a copy of the valid original CITES document, that designates the name of the importer in the country of final destination and is issued by the Management Authority of the exporting or re-exporting country. A copy of a CITES document is subject to verification.
(2) For shipment of an Appendix-I specimen, a copy of a valid import permit that designates the name of the importer in the country of final destination, unless the CITES document in paragraph (b)(1) of this section is a CITES exemption document (see § 23.20(d)).
(3) Transportation and routing documents that show the shipment has been consigned to the same importer and country of final destination as designated on the CITES document.
(c) Shipment requirements. An in-transit shipment, including items in an on-board store, must meet the following:
(1) When in an intermediary country, an in-transit shipment must stay only for the time needed to immediately transfer the specimen to the mode of transport used to continue to the final destination and remain under customs control. Other than during immediate transfer, the specimen may not be stored in a duty-free, bonded, or other kind of warehouse or a free trade zone.
(2) At any time during transit, an in-transit shipment must not be sold, manipulated, or split unless authorized by the Management Authority of the intermediary country for inspection or enforcement purposes.
(d) Reserving Party or non-Party. All the requirements of this section apply to shipments to or from a reserving Party or non-Party that are being transshipped through a Party. The CITES document must treat the specimen as listed in the Appendix as provided in § 23.21(d).
(e) Specimen protected by other regulations. Shipment of a specimen that is also listed as a migratory bird (part 10 of this subchapter), injurious wildlife (part 16 of this subchapter), endangered or threatened species (parts 17 of this subchapter and 222-224 of this title), marine mammal (parts 18 of this subchapter and 216 of this title), or bald or golden eagle (part 22 of this subchapter), and is moving through the United States is considered an import, and cannot be treated as an in-transit shipment (see § 23.3).
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.23 What information is required on U.S. and foreign CITES documents?
(a) Purpose. Article VI of the Treaty provides standard information that must be on a permit and certificate issued under Articles III, IV, and V. To identify a false or invalid document, any CITES document, including a CITES exemption document issued under Article VII, must contain standardized information to allow a Party to verify that the specimen being shipped is the one listed on the document and that the trade is consistent with the provisions of the Treaty.
(b) CITES form. A CITES document issued by a Party must be issued in one or more of the three working languages of CITES (English, Spanish, or French). A CITES document from a non–Party may be in the form of a permit or certificate, letter, or any other form that clearly indicates the nature of the document and includes the information in paragraphs (c) through (e) of this section and the additional information in § 23.25.
(c) Required information. Except for a phytosanitary certificate used as a CITES certificate for artificially propagated plants in paragraph (f) of this section, or a customs declaration label used to identify specimens being moved between registered scientific institutions (§ 23.48(e)(5)), a CITES document issued by a Party or non–Party must contain the information set out in this paragraph (listed alphabetically). Specific types of CITES documents must also contain the additional information identified in paragraph (e) of this section. A CITES document is valid only when it contains the following information:
|
Required information |
Description |
|
(1) Appendix |
The CITES Appendix in which the species, subspecies, or population is listed (see § 23.21 when a Party has taken a reservation on a listing). For products that contain or consist of more than one CITES species, the Appendix in which each species is listed must be indicated on the CITES document. |
|
(2) Applicant's signature |
The applicant's signature if the CITES document includes a place for it. |
|
(3) Bill of lading, air waybill, or flight number |
As applicable for export or re-export: (i) by ocean or air cargo, the bill of lading or air waybill number or (ii) in accompanying baggage, the flight number, as recorded on the CITES document by the inspecting official at the port, if known at the time of validation or certification. |
|
(4) Dates |
Date of issue and date of expiration (“valid until” date on the standardized CITES form), which is midnight of the date on the CITES document. See § 23.54 for the length of validity for different types of CITES documents. |
|
(5) Description of the specimen |
A complete description of the specimen, including whether live or the type of goods. The sex and age of a live specimen should be recorded, if possible. Such information must be in English, Spanish, or French on a CITES document from a Party. If a code is used to indicate the type of specimen, it must agree with the Guidelines for preparation and submission of CITES annual reports available from the CITES website or us (see § 23.7). |
|
(6) Document number |
A unique control number. We use a unique 12-character number. The first two characters are the last two digits of the year of issuance, the next two are the two-letter ISO country code, followed by a six-digit serial number, and two digits or letters used for national informational purposes. |
|
(7) Humane transport of live specimens |
If the CITES document authorizes the export or re-export of live specimens, a statement that the document is valid only if the transport conditions comply with the International Air Transport Association Live Animals Regulations or the International Air Transport Association Perishable Cargo Regulations (incorporated by reference, see § 23.9). A shipment containing live animals must comply with the requirements of the Live Animals Regulations (LAR). A shipment containing live plants must comply with the requirements for plants in the Perishable Cargo Regulations (PCR). |
|
(8) Identification of the specimen |
Any unique identification number or mark (such as a tag, band, ring, microchip, label, or serial number), including any mark required under these regulations or a CITES listing annotation. For a microchip, the microchip code, trademark of the transponder manufacturer and, where possible, the location of the microchip in the specimen. If a microchip is used, we may, if necessary, ask the importer, exporter, or re-exporter to have equipment on hand to read the microchip at the time of import, export, or re-export. |
|
(9) Management Authority |
The complete name and address of the issuing Management Authority as included in the CITES directory, which is available from the CITES website or us (see § 23.7). |
|
(10) Name and address |
The complete name and address, including country, of the exporter and importer. |
|
(11) Purpose of transaction |
The purpose of the transaction identified either through a written description of the purpose of the transaction or by using one of the codes given in paragraph (d) of this section. The code is determined by the issuing Management Authority through information submitted with an application. This is not required for a certificate of origin. |
|
(12) Quantity |
The quantity of specimens authorized in the shipment and, if appropriate, the unit of measurement using the metric system. For products that contain or consist of more than one CITES species, the quantity of each species must be indicated on the CITES document. |
|
(i) The unit of measurement should be appropriate to the type of specimen and agree with the Guidelines for the preparation and submission of CITES annual reports available from the CITES website or us (see § 23.7). General descriptions such as “one case” or “one batch” are not acceptable. |
|
|
(ii) Weight should be in kilograms. If weight is used, net weight (weight of the specimen alone) must be stated, not gross weight that includes the weight of the container or packaging. |
|
|
(iii) Volume should be in cubic meters for logs and sawn wood and either square meters or cubic meters for veneer and plywood. |
|
|
(iv) For re-export, if the type of good has not changed since being imported, the same unit of measurement as on the export permit must be used, except to change to units that are to be used in the CITES annual report. |
|
|
(13) Scientific name |
The scientific name of the species, including the subspecies when needed to determine the level of protection of the specimen under CITES. For products that contain or consist of more than one CITES species, the scientific name of each species must be indicated on the CITES document. Scientific names must be in the standard nomenclature as it appears in the CITES Appendices or the references adopted by the CoP. A list of current references is available from the CITES website or us (see § 23.7). A CITES document may contain higher-taxon names in lieu of the species name only under one of the following circumstances: |
|
(i) The CoP has agreed that the use of a higher-taxon name is acceptable for use on CITES documents. |
|
(A) If the genus cannot be readily determined for coral rock, the scientific name to be used is the order Scleractinia. |
||
|
(B) If the species cannot be determined for worked specimens of black coral, specimens may be identified at the genus level. If the genus cannot be determined for worked specimens of black coral, the scientific name to be used is the order Antipatharia. Raw black coral and live black coral must be identified to the level of species. |
||
|
(C) Live and dead coral must be identified to the level of species except where the CoP has agreed that identification to genus is acceptable. A current list of coral taxa identifiable to genus is available from the CITES website or us (see § 23.7). |
||
|
(D) Re-export of worked skins or pieces of Tupinambis species that were imported before August 1, 2000, may indicate Tupinambis spp. |
|
(ii) The issuing Party can show the use of a higher-taxon name is well justified and has communicated the justification to the Secretariat. |
|
|
(iii) The item is a pre-Convention manufactured product containing a specimen that cannot be identified to the species level. |
|
|
(14) Seal or stamp |
The embossed seal or ink stamp of the issuing Management Authority. |
|
(15) Security stamp |
If a Party uses a security stamp, the stamp must be canceled by an authorized signature and a stamp or seal, preferably embossed. The number of the stamp must also be recorded on the CITES document. |
|
(16) Signature |
An original handwritten signature or signature stamp of a person authorized to sign CITES documents for the issuing Management Authority. The signature must be on file with the Secretariat. |
|
(17) Signature name |
The name of the person who signed the CITES document. |
|
(18) Source |
The source of the specimen. For products that contain or consist of more than one CITES species, the source code of each species must be indicated on the CITES document. For re-export, unless there is information to indicate otherwise, the source code on the CITES document used for import of the specimen must be used. See § 23.24 for a list of codes. |
|
(19) Treaty name |
Either the full name or acronym of the Treaty, or the CITES logo. |
|
(20) Type of CITES document |
The type of CITES document (import, export, re-export, or other): |
|
(i) If marked “other,” the CITES document must indicate the type of document, such as certificate for artificially propagated plants, certificate for wildlife bred in captivity, certificate of origin, certificate of ownership, introduction-from-the-sea certificate, pre-Convention certificate, sample collection covered by an ATA carnet, scientific exchange certificate, or traveling-exhibition certificate. |
|
|
(ii) If multiple types are authorized on one CITES document, the type that applies to each specimen must be clearly indicated. |
|
|
(21) Validation or certification |
Except as provided for replacement (§ 23.52(f)) or retrospective (§ 23.53(f)) CITES documents, the actual quantity of specimens exported or re-exported: |
|
(i) Using the same units of measurement as those on the CITES document. |
|
|
(ii) Validated or certified by the stamp or seal and signature of the inspecting authority at the time of export or re-export. |
(d) Purpose of transaction. If the purpose is not identified by a written description, the CITES document must contain one of the following codes:
|
Code |
Purpose of transaction |
|
B
|
Breeding in captivity or artificial propagation |
|
E
|
Education |
|
G
|
Botanical garden |
|
H
|
Hunting trophy |
|
L
|
Law enforcement/judicial/forensic |
|
M
|
Medical research (including biomedical research) |
|
N
|
Reintroduction or introduction into the wild |
|
P
|
Personal |
|
Q
|
Circus and traveling exhibition |
|
S
|
Scientific |
|
T
|
Commercial |
|
Z
|
Zoo |
|
Type of document |
Additional required information |
|
(1) Annex (such as an attached inventory, conditions, or continuation pages of a CITES document) |
The page number, document number, and date of issue on each page of an annex that is attached as an integral part of a CITES document. An authorized signature and ink stamp or seal, preferably embossed, of the Management Authority issuing the CITES document must also be included on each page of the annex. The CITES document must indicate an attached annex and the total number of pages. |
|
(2) Certificate of origin (see § 23.38) |
A statement that the specimen originated in the country that issued the certificate. |
|
(3) Copy when used in place of the original CITES document |
(i) Information required in paragraph (e)(7) of this section when the document authorizes export or re-export. |
|
(ii) A statement by the Management Authority on the face of the document authorizing the use of a copy when the document authorizes import. |
|
|
(4) Export permit for a registered commercial breeding operation or nursery for Appendix-I specimens (see § 23.46) |
The registration number of the operation or nursery assigned by the Secretariat, and if the exporter is not the registered operation or nursery, the name of the registered operation or nursery. |
|
(5) Export permit with a quota |
Number of specimens, such as 500/1,000, that were: |
|
(i) Exported thus far in the current year, including those covered by the current permit (such as 500), and |
|
|
(ii) Included in the current annual quota (such as 1,000). |
|
|
(6) Import permit (Appendix-I specimen) (see § 23.35) |
A certification that the specimen will not be used for primarily commercial purposes and, for a live specimen, that the recipient has suitable facilities and expertise to house and care for it. |
|
(7) Replacement CITES document (see § 23.52) |
When a CITES document replaces an already issued CITES document that was lost, damaged, stolen, or accidentally destroyed: |
|
(i) If a newly issued CITES document, indication it is a “replacement,” the number and date of issuance of the CITES document that was replaced, and reason for replacement. |
|
|
(ii) If a copy of the original CITES document, indication it is a “replacement” and a “true copy of the original,” a new original signature of a person authorized to sign CITES documents for the issuing Management Authority, the date signed, and reason for replacement. |
|
|
(8) Partially completed documents (see § 23.51) |
(i) A list of the blocks that must be completed by the permit holder. |
|
(ii) If the list includes scientific names, an inventory of approved species must be included on the face of the CITES document or in an attached annex. |
|
|
(iii) A signature of the permit holder, which acts as a certification that the information entered is true and accurate. |
|
|
(9) Pre-Convention document (see § 23.45) |
(i) An indication on the face of the CITES document that the specimen is pre-Convention. |
|
(ii) A date that shows the specimen was acquired before the date the Convention first applied to it. |
|
|
(10) Re-export certificate (see § 23.37) |
(i) The country of origin, the export permit number, and the date of issue. |
|
(ii) If previously re-exported, the country of last re-export, the re-export certificate number, and the date of issue. |
|
|
(iii) If all or part of this information is not known, a justification must be given. |
|
|
(iv) For products that contain or consist of more than one CITES species, the information in paragraphs (e)(10)(i) through (iii) of this section for each species must be indicated on the CITES document. |
|
|
(11) Retrospective CITES document (see § 23.53) |
A clear statement that the CITES document is issued retrospectively and the reason for issuance. |
|
(12) Sample collection covered by an ATA carnet (see § 23.50) |
(i) A statement that the document covers a sample collection and is invalid unless accompanied by a valid ATA carnet. |
|
(ii) The number of the accompanying ATA carnet recorded by the Management Authority, customs, or other responsible CITES inspecting official. |
Credits
[79 FR 30422, May 27, 2014; 79 FR 32677, June 6, 2014
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.24 What code is used to show the source of the specimen?
The Management Authority must indicate on the CITES document the source of the specimen using one of the following codes, except the code “O” for pre–Convention, which may be used in conjunction with another code:
|
Source of specimen |
Code |
||
|
(a) Artificially propagated plant (see § 23.40): |
A |
||
|
(1) An Appendix-II or -III artificially propagated specimen. |
|||
|
(2) An Appendix-I plant specimen artificially propagated for noncommercial purposes or certain Appendix-I hybrids (see § 23.42) propagated for commercial purposes. |
|||
|
(b) Bred-in-captivity wildlife (see § 23.41): |
C |
||
|
(1) An Appendix-II or -III specimen bred in captivity. (See paragraph (d)(1) of this section for wildlife that does not qualify as bred in captivity.) |
|||
|
(2) An Appendix-I specimen bred for noncommercial purposes. (See paragraph (c)(1) of this section for an Appendix-I specimen bred for commercial purposes.) |
|||
|
(c) Bred in captivity or artificially propagated for commercial purposes (see §§ 23.46 and 23.47): |
D |
||
|
(1) An Appendix-I wildlife specimen bred in captivity for commercial purposes at an operation registered with the Secretariat. |
|||
|
(2) An Appendix-I plant specimen artificially propagated for commercial purposes at a nursery that is registered with the Secretariat or a commercial propagating operation that meets the requirements of § 23.47. |
|||
|
(d) Captive-bred wildlife (§ 23.36): |
F |
||
|
(1) An Appendix-II or -III wildlife species that is captive-bred. |
|||
|
(2) An Appendix-I wildlife species that is one of the following: |
|||
|
(i) Captive-bred (see § 23.5). |
|||
|
(ii) Bred for commercial purposes, but the commercial breeding operation is not registered with the Secretariat. |
|||
|
(e) Confiscated or seized specimen (see § 23.78). |
I |
||
|
(f) Pre-Convention specimen (see § 23.45) (code may be used in conjunction with another code). |
O |
||
|
(g) Ranched wildlife (see § 23.5). |
R |
||
|
(h) Source unknown (must be justified on the face of the CITES document). |
U |
||
|
(i) Specimen taken from the wild: |
W |
||
|
(1) For wildlife, this includes a specimen born in captivity from an egg collected from the wild or from wildlife that mated or exchanged genetic material in the wild. |
|||
|
(2) For a plant, it includes a specimen propagated from a propagule collected from a wild plant, except as provided in § 23.64. |
|||
Credits
[79 FR 30423, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
§ 23.25 What additional information is required on a non–Party CITES document?
(a) Purpose. Under Article X of the Treaty, a Party may accept a CITES document issued by a competent authority of a non–Party only if the document substantially conforms to the requirements of the Treaty.
(b) Additional certifications. In addition to the information in § 23.23(c) through (e), a CITES document issued by a non–Party must contain the following certifications on the face of the document:
|
Activity by a non-Party
|
Certification
|
|
(1) Export
|
(i) For Appendix-I and -II specimens, the Scientific Authority has advised that the export will not be detrimental to the survival of the species.
|
|
(ii) The Management Authority is satisfied that the specimen was legally acquired.
|
|
|
(2) Import
|
For Appendix-I specimens, the import will be for purposes that are not detrimental to the survival of the species.
|
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.26 When is a U.S. or foreign CITES document valid?
(a) Purpose. Article VIII of the Treaty provides that Parties take appropriate measures to enforce the Convention to prevent illegal trafficking in wildlife and plants.
(b) Original CITES documents. A separate original or a true copy of a CITES document must be issued before the import, introduction from the sea, export, or re-export occurs, and the document must accompany each shipment. No copy may be used in place of an original except as provided in § 23.23(e)(3) or when a shipment is in transit (see § 23.22). Fax or electronic copies are not acceptable.
(c) Acceptance of CITES documents. We will accept a CITES document as valid for import, introduction from the sea, export, or re-export only if the document meets the requirements of this section, §§ 23.23 through 23.25, and the following conditions:
|
Key phrase |
Conditions for an acceptable CITES document |
|
(1) Altered or modified CITES document |
The CITES document has not been altered (including by rubbing or scratching out), added to, or modified in any way unless the change is validated on the document by the stamp and authorized signature of the issuing Management Authority, or if the document was issued as a partially completed document, the Management Authority lists on the face of the document which blocks must be completed by the permit holder. |
|
(2) Annual reports |
The Party issuing the CITES document has submitted annual reports and is not subject to any action under Article VIII paragraph 7(a) that would not allow trade in CITES species. |
|
(3) CITES document |
U.S. and foreign CITES documents must meet the general provisions and criteria in subparts C and E. |
|
(4) Conditions |
All conditions on the CITES document are met. |
|
(5) Convention implementation |
The Party issuing the CITES document is not subject to any action under Article VIII or Article XIII paragraph 3 that would not allow trade in the species. |
|
(6) Extension of validity |
The validity of a CITES document may not be extended except as provided in § 23.73 for certain timber species. |
|
(7) Fraudulent CITES document or CITES document containing false information |
The CITES document is authentic and does not contain erroneous or misleading information. |
|
(8) Humane transport |
Live wildlife or plants were transported in compliance with the International Air Transport Association Live Animals Regulations (for animals) or the International Air Transport Association Perishable Cargo Regulations (for plants) (incorporated by reference, see § 23.9). |
|
(9) Legal acquisition |
The Party or non-Party issuing the CITES document has made the required legal acquisition finding. |
|
(10) Management Authority and Scientific Authority |
The CITES document was issued by a Party or non-Party that has designated a Management Authority and Scientific Authority and has provided information on these authorities to the Secretariat. |
|
(11) Name of importer and exporter |
A CITES document is specific to the name on the face of the document and may not be transferred or assigned to another person. |
|
(12) Non-detriment |
The Party or non-Party issuing the CITES document has made the required non-detriment finding. |
|
(13) Phytosanitary certificate |
A phytosanitary certificate may be used to export artificially propagated plants only if the issuing Party has provided copies of the certificates, stamps, and seals to the Secretariat. |
|
(14) Quota |
For species with a quota on file with the Secretariat, the quantity exported from a country does not exceed the quota. |
|
(15) Registered |
(i) The operation is included in the Secretariat's register. |
|
commercial breeding operation for Appendix-I wildlife |
(ii) Each specimen is specifically marked, and the mark is described on the CITES document. |
|
(16) Registered commercial nursery for Appendix-I plants |
The operation is included in the Secretariat's register. |
|
(17) Retrospective CITES documents |
A CITES document was not issued retrospectively except as provided in § 23.53. |
|
(18) Shipment contents |
The contents of the shipment match the description of specimens provided on the CITES document, including the units and species. A shipment cannot contain more or different specimens or species than certified or validated on the CITES document at the time of export or re-export; the quantity of specimens validated or certified may be less, but not more, than the quantity stated at the time of issuance. |
|
(19) Wild-collected specimen |
A wild-collected specimen (indicated on the CITES document with a source code of “W”) is not coming from a country that is outside the range of the species, unless we have information indicating that the species has been established in the wild in that country through accidental introduction or other means. |
(d) Verification of a CITES document. We may request verification of a CITES document from the Secretariat or a foreign Management Authority before deciding whether to accept it under some circumstances, including, but not limited to, the following:
(1) We receive reliable information that indicates the need for CITES document verification.
(2) We have reasonable grounds to believe that a CITES document is not valid or authentic because the species is being traded in a manner detrimental to the survival of the species or in violation of foreign wildlife or plant laws, or any applicable Management or Scientific Authority finding has not been made.
(3) The re-export certificate refers to an export permit that does not exist or is not valid.
(4) The CITES document includes a species for which the Secretariat has published an annotated quota.
(5) We have reasonable grounds to believe that the document is fraudulent, contains false information, or has unauthorized changes.
(6) We have reasonable grounds to believe that the specimen identified as bred in captivity or artificially propagated is a wild specimen, was produced from illegally acquired parental stock, or otherwise does not qualify for these exemptions.
(7) We know or have reasonable grounds to believe that an Appendix–I specimen was not bred at a facility registered with the CITES Secretariat and that the purpose of the import is commercial.
(8) The import of a specimen designated as bred in captivity or artificially propagated is from a non–Party. For an Appendix–I specimen, we must consult with the Secretariat.
(9) For a retrospectively issued CITES document, both the importing and exporting or re-exporting countries' Management Authorities have not agreed to the issuance of the document.
(10) For a replacement CITES document, we need clarification of the reason the document was issued.
(11) The export permit or re-export certificate does not contain validation or certification by an inspecting official at the time of export of the actual quantity exported or re-exported.
Credits
[79 FR 30423, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
§ 23.27 What CITES documents do I present at the port?
(a) Purpose. Article VIII of the Treaty provides that Parties establish an inspection process that takes place at a port of exit and entry. Inspecting officials must verify that valid CITES documents accompany shipments and take enforcement action when shipments do not comply with the Convention. Article VI, paragraph 6, of the Treaty requires that the Management Authority of the importing country cancel and retain the export permit or re-export certificate and any corresponding import permit presented. In the United States, for imports of CITES–listed plant specimens, CITES inspecting officials cancel and submit original CITES documents to the U.S. Management Authority.
(b) U.S. port requirements. In the United States, you must follow the clearance requirements for wildlife in part 14 of this subchapter and for plants in part 24 of this subchapter and 7 CFR parts 319, 352, and 355, and the specific requirement in paragraphs (c) and (d) of this section.
(c) General validation or certification process. Officials in each exporting or re-exporting country inspect the shipment and validate or certify the CITES document. The table in this paragraph (c) provides information on:
(1) The types of original CITES documents you must present to be validated or certified by the inspecting official to export or re-export from a country.
(2) When you need to surrender a copy of the original CITES document to the inspecting official at the time of export or re-export.
(3) When you need to surrender the original CITES document to the inspecting official at the time of import or introduction from the sea.
|
Type of CITES document |
Present original for export or re-export validation or certification |
Surrender copy upon export or re-export |
Surrender original upon import or introduction from the sea |
|
Bred-in-captivity certificate |
Required |
Required |
Required |
|
Certificate for artificially propagated plants |
Required |
Required |
Required |
|
Certificate of origin |
Required |
Required |
Required |
|
Certificate of ownership |
Required |
Required |
Not required; submit copy |
|
Export permit |
Required |
Required |
Required |
|
Import permit |
Not required |
Required |
Required |
|
Introduction-from-the-sea certificate |
Not applicable |
Not applicable |
Required |
|
Multiple-use document |
Required1 |
Required |
Not required; submit copy |
|
Phytosanitary certificate |
Required |
Required |
Not required; submit copy |
|
Pre-Convention document |
Required |
Required |
Required |
|
Re-export certificate |
Required |
Required |
Required |
|
Registered Appendix-I commercial breeding operation, export permit |
Required |
Required |
Required |
|
Registered Appendix-I nursery, export permit |
Required |
Required |
Required |
|
Replacement document where a shipment has been made and is in a foreign country |
Not required |
Not required |
Required |
|
Replacement document where a shipment has not left the United States |
Required |
Required |
Required |
|
Retrospective document |
Not required |
Not required |
Required |
|
Sample collection covered by an ATA carnet, CITES document |
Required |
Required |
Not required; submit copy |
|
Traveling-exhibition certificate |
Required |
Required |
Not required; submit copy |
(d) Customs declaration labels. The customs declaration label used to identify specimens being moved between registered scientific institutions (§ 23.48) must be affixed to the shipping container. The label does not require export or re-export validation or certification at the port.
Credits
[79 FR 30424, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
Subpart C. Application Procedures, Criteria, and Conditions
§ 23.32 How do I apply for a U.S. CITES document?
(a) To apply for a U.S. CITES document, you must complete a standard application form and submit it to the appropriate office shown on the top of the form.
(b) To determine the type of CITES document needed for your shipment, go to §§ 23.18 through 23.20 for further guidance.
(c) If a species is also regulated under another part of this subchapter (such as endangered or threatened species, see § 23.3), the requirements of all parts must be met. You may submit a single application that contains all the information needed to meet the requirements of CITES and other applicable parts.
(d) You must also follow the general permit procedures in part 13 of this subchapter.
(e) You should review the criteria in all applicable regulations in this subchapter that apply to the type of permit you are seeking before completing the application form.
(f) We will review your application to assess whether it contains the information needed to make the required findings.
(1) Based on available information, we will decide if any of the exemptions apply and what type of CITES document you need.
(2) If we need additional information, we will contact you. If you do not provide the information within 45 calendar days, we will abandon your application. If your application is abandoned and you wish to apply for a permit at a later time, you must submit a new application.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.33 How is the decision made to issue or deny a request for a U.S. CITES document?
(a) Upon receiving a complete application, we will decide whether to issue a CITES document by considering:
(1) The general criteria in § 13.21(b) of this subchapter and, if the species is protected under a separate law or treaty, criteria in any other applicable parts.
(2) The CITES issuance criteria provided in this subpart (see subpart D of this part for factors we consider in making certain findings).
(b) As needed, the U.S. Management Authority, including FWS Law Enforcement, will forward a copy of the application to the U.S. Scientific Authority; State, tribal, or other Federal government agencies; or other applicable experts. We may also query the Secretariat and foreign Management and Scientific Authorities for information to use in making the required findings.
(c) You must provide sufficient information to satisfy us that all criteria specific to the proposed activity are met before we can issue a CITES document.
(d) We will base our decision on whether to issue or deny the application on the best available information.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.34 What kinds of records may I use to show the origin of a specimen when I apply for a U.S. CITES document?
(a) When you apply for a U.S. CITES document, you will be asked to provide information on the origin of the specimen that will be covered by the CITES document.
(1) You need to provide sufficient information for us to determine if the issuance criteria in this part are met (see the sections in this subpart for each type of CITES document).
(2) We require less detailed information when the import, introduction from the sea, export, or re-export poses a low risk to a species in the wild and more detailed information when the proposed activity poses greater risk to a species in the wild (see Subpart D of this part for factors we consider in making certain findings).
(b) Information you may want to provide in a permit application includes, but is not limited to, the following:
|
Source of specimen |
Types of records |
|
|
(1) Captive-bred or cultivated 1
|
(i) Records that identify the breeder or propagator of the specimens that have been identified by birth, hatch, or propagation date and for wildlife by sex, size, band number, or other mark, or for plants by size or other identifying feature: |
|
|
|
(A) Signed and dated statement by the breeder or propagator that the specimen was bred or propagated under controlled conditions. |
|
|
(B) Name and address of the breeder or propagator as shown by documents such as an International Species Information System (ISIS) record, veterinary certificate, or plant nursery license. |
||
|
(ii) Records that document the breeding or propagating of specimens at the facility: |
||
|
(A) Number of wildlife (by sex and age- or size-class) or plants at the facility. |
||
|
(B) How long the facility has been breeding or propagating the species. |
||
|
(C) Annual production and mortalities. |
||
|
(D) Number of specimens sold or transferred annually. |
||
|
(E) Number of specimens added from other sources annually. |
||
|
(F) Transaction records with the date, species, quantity of specimens, and name and address of seller. |
||
|
(G) Marking system, if applicable. |
||
|
(H) Photographs or video of facility, including for wildlife any activities during nesting and production and rearing of young, and for plants, different stages of growth. |
||
|
(2) Confiscated or seized
|
Copy of remission decision, legal settlement, or disposal action after forfeiture or abandonment, which demonstrates the applicant's legal possession. |
|
|
(3) Grown from exempt plant material
|
Records that document how you obtained the exempt plant material, including the name and address of the person from whom you received the plant material. |
|
|
(4) Imported previously
|
(i) A copy of the cancelled CITES document that accompanied the shipment into the United States. |
|
|
(ii) For wildlife, copies of cleared Declarations for Importation or Exportation of Fish or Wildlife (Form 3-177) associated with each specimen. |
||
|
(5) Pre-Convention
|
Records that show the specimen was acquired before the date the provisions of the Convention first applied to it, such as: |
|
|
(i) Receipt or invoice. |
||
|
(ii) Catalog, inventory list, photograph, or art book. |
||
|
(iii) Statement from a qualified appraiser attesting to the age of a manufactured product. |
||
|
(iv) CBP (formerly U.S. Customs Service) import documents. |
||
|
(v) Phytosanitary certificate. |
||
|
(vi) Veterinary document or breeding or propagation logs. |
||
|
(6) Ranched wildlife
|
(i) Records, such as permits, licenses, and tags, that demonstrate that the specimen was legally removed from the wild under relevant Federal, tribal, State, or local wildlife conservation laws or regulations: |
|
|
|
(A) If taken on private or tribal land, permission of the landowner if required under applicable law. |
|
|
|
(B) If taken in a national, State, or local park, refuge or other protected area, permission from the applicable agency, if required. |
|
|
|
(ii) Records that document the rearing of specimens at the facility: |
|
|
|
(A) Number of specimens (by sex and age- or size-class) at the facility. |
|
|
|
(B) How long the specimens were reared at the facility. |
|
|
|
(C) Signed and dated statement by the owner or manager of the facility that the specimens were reared at the facility in a controlled environment. |
|
|
|
(D) Marking system, if applicable. |
|
|
|
(E) Photographs or video of the facility. |
|
|
(7) Sequential ownership or purchase
|
(i) Records that specifically identify the specimen, give the name and address of the owner, and show the specimen's origin (pre-Convention, previously imported, wild-collected, or born or propagated in a controlled environment in the United States). |
|
|
(ii) Records that document the history of all transfers in ownership (generally not required for pre-Convention specimens). |
||
|
(8) Unknown origin, for noncommercial purposes
|
A complete description of the circumstances under which the specimen was acquired (where, when, and from whom the specimen was acquired), including efforts made to obtain information on the origin of the specimen. |
|
|
(9) Wild-collected
|
Records, such as permits, licenses, and tags, that demonstrate the specimen or the parental stock was legally removed from the wild under relevant foreign, Federal, tribal, State, or local wildlife or plant conservation laws or regulations: |
|
|
(i) If taken on private or tribal land, permission of the landowner if required under applicable law. |
||
|
(ii) If taken in a national, State, or local park, refuge, or other protected area, permission from the applicable agency, if required. |
||
(c) If you intend to engage in international trade with a CITES specimen in the future, you should keep sufficient records to establish your eligibility for a CITES document for as long as you possess the specimen, and if you sell, donate, or transfer ownership of the specimen, you should provide such records on the origin of the specimen to the new owner.
Credits
[79 FR 30424, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.35 What are the requirements for an import permit?
(a) Purpose. Article III(3) of the Treaty sets out the conditions under which a Management Authority can issue an import permit.
(b) U.S. application forms. Complete the appropriate form for the proposed activity and submit it to the U.S. Management Authority:
|
Type of application for an import permit for an Appendix-I specimen
|
Form no.
|
|
(1)
|
CITES:
|
|
|
Southern African Leopard, African Elephant, and Namibian Southern White Rhinoceros Sport-hunted Trophies
|
3-200-19
|
|
|
Appendix-I Plants
|
3-200-35
|
|
|
Appendix-I Wildlife
|
3-200-37
|
|
|
Appendix-I Biological Samples
|
3-200-29
|
|
|
(2)
|
Endangered Species Act and CITES:
|
|
|
ESA Plants
|
3-200-36
|
|
|
ESA Sport-hunted Trophies
|
3-200-20
|
|
|
ESA Wildlife
|
3-200-37
|
|
|
(3)
|
Marine Mammal Protection Act and CITES:
|
|
|
Marine Mammals
|
3-200-43
|
|
|
(4)
|
Wild Bird Conservation Act and CITES:
|
|
|
Personal Pet Bird
|
3-200-46
|
|
|
Under an Approved Cooperative Breeding Program
|
3-200-48
|
|
|
Scientific Research or Zoological Breeding/Display
|
3-200-47
|
|
Criteria for an import permit for an Appendix-I specimen
|
Section
|
|
(1) The proposed import would be for purposes that are not detrimental to the survival of the species.
|
23.61
|
|
(2) The specimen will not be used for primarily commercial purposes.
|
23.62
|
|
(3) The recipients are suitably equipped to house and care for any live wildlife or plant to be imported.
|
23.65
|
|
(4) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP.
|
23.23
|
(d) U.S. standard conditions. You must meet all of the provisions on use after import in § 23.55 and the standard conditions in § 23.56.
(e) Prior issuance of an import permit. For Appendix–I specimens, the Management Authority of the exporting country may:
(1) Issue an export permit for live or dead specimens or a re-export certificate for live specimens only after the Management Authority of the importing country has either issued an import permit or confirmed in writing that an import permit will be issued.
(2) Accept oral confirmation from the Management Authority of the importing country that an import permit will be issued in an emergency situation where the life or health of the specimen is threatened and no means of written communication is possible.
(3) Issue a re-export certificate for a dead specimen without confirmation that the import permit has been issued.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.36 What are the requirements for an export permit?
(a) Purposes. Articles III, IV, and V of the Treaty set out the conditions under which a Management Authority may issue an export permit for an Appendix–I, -II, or -III specimen. Article XIV sets out the conditions under which a Management Authority may issue a document for export of certain Appendix–II marine specimens protected under a pre-existing treaty, convention, or international agreement.
(b) U.S. application forms. Complete the appropriate form for the proposed activity and submit it to the U.S. Management Authority. Form 3–200–26 may also be submitted to FWS Law Enforcement at certain ports or regional offices:
|
Type of application for an export permit |
Form no. |
|
(1) |
CITES: |
|
|
American Ginseng |
3-200-34 |
|
|
Appendix-I Plants Artificially Propagated for Commercial Purposes |
3-200-33 |
|
|
Biological Specimens |
3-200-29 |
|
|
Captive-born Raptors |
3-200-25 |
|
|
Captive-born Wildlife (except raptors) |
3-200-24 |
|
|
Caviar/Live Eggs/Meat of Paddlefish or Sturgeon, From an Aquaculture Facillity |
3-200-80 |
|
|
Caviar/Meat of Paddlefish or Sturgeon, Removed from the Wild |
3-200-76 |
|
|
Export of Skins of Bobcat, Canada Lynx, River Otter, Brown Bear, Gray Wolf, and American Alligator Taken under an Approved State or Tribal Program |
3-200-26 |
|
|
Master File for the Export of Live Animals Bred in Captivity |
3-200-85 |
|
|
Personal Pets, One-time Export |
3-200-46 |
|
|
Plants |
3-200-32 |
|
|
Registration of a Native Species Production Facility |
3-200-75 |
|
|
Single-use Permits under a Master File or an Annual Program File |
3-200-74 |
|
|
Trophies by Hunters or Taxidermists |
3-200-28 |
|
|
Wildlife, Removed from the Wild “(Live Animals/Samples/Parts/Products) |
3-200-27 |
|
|
(2) |
Endangered Species Act and CITES: |
|
|
ESA Plants |
3-200-36 |
|
|
ESA Wildlife |
3-200-37 |
|
|
(3) |
Marine Mammal Protection Act and CITES: |
|
|
Biological Samples |
3-200-29 |
|
|
Live Captive-held Marine Mammals |
3-200-53 |
|
|
Take from the Wild for Export |
3-200-43 |
|
Appendix of the specimen |
||||
|
Criteria for an export permit |
I |
II |
III |
Section |
|
(1) The wildlife or plant was legally acquired. |
Yes |
Yes |
Yes |
23.60 |
|
(2) The proposed export would not be detrimental to the survival of the species. |
Yes |
Yes |
n/a |
23.61 |
|
(3) An import permit has already been issued or the Management Authority of the importing country has confirmed that it will be issued. |
Yes |
n/a |
n/a |
23.35 |
|
(4) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP. |
Yes |
Yes |
Yes |
23.23 |
|
(5) Live wildlife or plants will be prepared and shipped so as to minimize risk of injury, damage to health, or cruel treatment of the specimen. |
Yes |
Yes |
Yes |
23.23 |
|
(6) The specimen originated in a country that listed the species. |
n/a |
n/a |
Yes |
23.20 |
|
(7) For wildlife with the source code “W” or “F,” the export is for noncommercial purposes. (See § 23.46 for the export of specimens that originated at a commercial breeding operation for Appendix-I wildlife that is registered with the Secretariat.) |
Yes |
n/a |
n/a |
- |
(d) Export of certain exempt marine specimens. Article XIV(4) and (5) of the Treaty provide a limited exemption for Appendix–II marine species that are protected under another treaty, convention, or international agreement that was in force at the time CITES entered into force. When all of the following conditions are met, export of exempt Appendix–II marine wildlife or plants requires only that the shipment is accompanied by a document issued by the Management Authority of the exporting country indicating that the specimens were taken in accordance with the provisions of the other international treaty, convention, or agreement:
(1) The exporting country is a CITES Party and is a party to an international treaty, convention, or agreement that affords protection to the species and was in force on July 1, 1975.
(2) The ship that harvested the specimen is registered in the exporting country.
(3) The specimen was taken within waters under the jurisdiction of the exporting country or in the marine environment not under the jurisdiction of any country.
(4) The specimen was taken in accordance with the other international treaty, convention, or agreement, including any quotas.
(5) The shipment is accompanied by any official document required under the other international treaty, convention, or agreement or otherwise required by law.
(e) Export of exempt specimens from the United States. To export a specimen exempted under paragraph (d) of this section, you must obtain a CITES document from the U.S. Management Authority that indicates the specimen was taken in accordance with the provisions of another international treaty, convention, or agreement that was in force on July 1, 1975.
(f) U.S. application for export of exempt specimens. To apply for a CITES exemption document under paragraph (e) of this section, complete the appropriate form for your activity and submit it to the U.S. Management Authority.
(g) Criteria for certain exempt marine specimens. The criteria in this paragraph (g) apply to the issuance and acceptance of U.S. and foreign export documents. To obtain a U.S. CITES document for export of specimens exempted under paragraph (d) of this section you must provide sufficient information for us to find that your proposed export meets all of the following issuance criteria:
(1) The specimen was taken in accordance with the provisions of an applicable international treaty, convention, or agreement that was in force on July 1, 1975.
(2) The scientific name of the CITES species is in the standard nomenclature in the CITES Appendices or references adopted by the CoP (see § 23.23).
(3) The ship that harvested the specimen is registered in the exporting country.
(4) The specimen was taken within waters under the jurisdiction of the exporting country or in the marine environment not under the jurisdiction of any country.
Credits
[79 FR 30424, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.37 What are the requirements for a re-export certificate?
(a) Purposes. Articles III, IV, and V of the Treaty set out the conditions under which a Management Authority may issue a re-export certificate for an Appendix–I, -II, or -III specimen.
(b) U.S. application forms. Complete the appropriate form for the proposed activity and submit it to the U.S. Management Authority. Form 3–200–73 may also be submitted to Law Enforcement at certain ports or regional offices:
|
Type of application for a re-export certificate
|
Form no.
|
|
|
(1)
|
CITES:
|
|
|
Biological Specimens
|
3-200-29
|
|
|
Plants
|
3-200-32
|
|
|
Single-use Permits under a Master File or an Annual Program File
|
3-200-74
|
|
|
Trophies by Taxidermists
|
3-200-28
|
|
|
Wildlife
|
3-200-73
|
|
|
(2)
|
Endangered Species Act and CITES:
|
|
|
ESA Plants
|
3-200-36
|
|
|
ESA Wildlife
|
3-200-37
|
|
|
(3)
|
Marine Mammal Protection Act and CITES:
|
|
|
Biological Samples
|
3-200-29
|
|
|
Live Captive-held Marine Mammals
|
3-200-53
|
|
|
Appendix of the specimen
|
||||
|
Criteria for a re-export certificate
|
I
|
II
|
III
|
Section
|
|
(1) The wildlife or plant was legally acquired.
|
Yes
|
Yes
|
Yes
|
23.60
|
|
(2) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP.
|
Yes
|
Yes
|
Yes
|
23.23
|
|
(3) For a live specimen, an import permit has already been issued or the Management Authority of the importing country has confirmed that it will be issued. This criterion does not apply to a specimen with the source code “D.”
|
Yes
|
n/a
|
n/a
|
23.35
|
|
(4) Live wildlife or plants will be prepared and shipped so as to minimize risk of injury, damage to health, or cruel treatment of the specimen.
|
Yes
|
Yes
|
Yes
|
23.23
|
|
(5) For re-export of a confiscated specimen, the proposed re-export would not be detrimental to the survival of the species.
|
Yes
|
Yes
|
n/a
|
23.61
|
|
(6) For wildlife with the source code “W” or “F,” the re-export is for noncommercial purposes.
|
Yes
|
n/a
|
n/a
|
--
|
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.38 What are the requirements for a certificate of origin?
(a) Purpose. Article V(3) of the Treaty requires that a shipment of Appendix-III specimens be accompanied by a certificate of origin when the shipment is not from a country that listed the species in Appendix III and is not a re-export.
(b) U.S. application forms. For a certificate of origin, complete one of the following forms and submit it to the U.S. Management Authority:
(1) Form 3-200-27 for wildlife removed from the wild.
(2) Form 3-200-24 for captive-born wildlife.
(3) Form 3-200-32 for plants.
(c) Criteria. The criteria in this paragraph (c) apply to the issuance and acceptance of U.S. and foreign certificates of origin. When applying for a U.S. certificate, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
(1) The specimen originated in the country of export, which is not a country that listed the species in Appendix III. In the case of a listing that is annotated to cover only a certain population, no CITES document is required if the listed population does not occur in the country of export. For U.S. applicants, the country of origin must be the United States.
(2) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP (see § 23.23).
(3) Live wildlife or plants will be prepared and shipped so as to minimize risk of injury, damage to health, or cruel treatment of the specimen (see § 23.23).
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.39 What are the requirements for an introduction-from-the-sea certificate?
(a) Purpose. Articles III(5), IV(6), and IV(7) of the Treaty set out the conditions under which a Management Authority may issue an introduction-from-the-sea certificate.
(b) U.S. application form. Complete Form 3–200–31 and submit it to the U.S. Management Authority.
(c) Criteria. The criteria in this paragraph (c) apply to the issuance and acceptance of U.S. certificates. You must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
|
Appendix of the specimen
|
|||
|
Criteria for an introduction-from-the-sea certificate
|
I
|
II
|
Section
|
|
(1) The specimen was taken in the marine environment not under the jurisdiction of any country.
|
Yes
|
Yes
|
-
|
|
(2) The proposed introduction from the sea would not be detrimental to the survival of the species.
|
Yes
|
Yes
|
23.61
|
|
(3) The specimen will not be used for primarily commercial purposes.
|
Yes
|
n/a
|
23.62
|
|
(4) The recipients are suitably equipped to house and care for live wildlife or plants.
|
Yes
|
n/a
|
23.65
|
|
(5) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP.
|
Yes
|
Yes
|
23.23
|
|
(6) Live wildlife or plants will be prepared and shipped so as to minimize risk of injury, damage to health, or cruel treatment of the specimen.
|
Yes
|
Yes
|
23.23
|
(d) Exemption. As allowed under Article XIV(4) and (5) of the Treaty, you may directly introduce into the United States any Appendix–II wildlife or plant taken in the marine environment that is not under the jurisdiction of any country without a CITES document when all of the following conditions are met:
(1) The United States is a party to an international treaty, convention, or agreement that affords protection to the species and was in force on July 1, 1975.
(2) The ship that harvested the specimen is registered in the United States.
(3) The specimen was taken in accordance with the other international treaty, convention, or agreement, including any quotas.
(4) The shipment is accompanied by any official document required under the other international treaty, convention, or agreement or otherwise required by U.S. law.
(e) Export of exempt specimens. To export a specimen exempted under paragraph (d) of this section, you must obtain a CITES document from the U.S. Management Authority that indicates the specimen was taken in accordance with the provisions of the other international treaty, convention, or agreement that was in force on July 1, 1975. See requirements in § 23.36 (e) through (g).
(f) Appendix III. Appendix–III species introduced from the sea do not require introduction-from-the-sea certificates. However, the subsequent international trade of an Appendix–III specimen introduced from the sea would be considered an export requiring a CITES document (see § 23.20(f)).
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.40 What are the requirements for a certificate for artificially propagated plants?
((a) Purpose. Article VII(5) of the Treaty grants an exemption to plants that are artificially propagated when a Management Authority issues a certificate.
(b) U.S. and foreign general provisions. The following provisions apply to the issuance and acceptance of a certificate for artificially propagated Appendix–I, -II, or -III plants:
(1) The certificate for artificially propagated plants and any subsequent re-export certificate must show the source code as “A” for artificially propagated.
(2) For an Appendix–I specimen that satisfies the requirements of this section, no CITES import permit is required.
(c) U.S. application form. Complete Form 3–200–33 and submit it to the U.S. Management Authority.
(d) Criteria. The criteria in this paragraph (d) apply to the issuance and acceptance of U.S. and foreign certificates. When applying for a U.S. certificate, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
|
Appendix of the specimen |
|||||
|
Criteria for a certificate for artificially propagated plants |
I |
II |
III |
Section |
|
|
(1) The plant was artificially propagated. |
Yes |
Yes |
Yes |
23.64 |
|
|
(2) The plant specimen is one of the following: |
Yes |
n/a |
n/a |
||
|
(i) Was propagated for noncommercial purposes. |
|||||
|
(ii) Is part of a traveling exhibition. |
|||||
|
(iii) Is a hybrid of one or more Appendix-I species or taxa that is not annotated to treat hybrids as Appendix–I specimens and was propagated for commercial or noncommercial purposes. |
|||||
|
(3) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP. |
Yes |
Yes |
Yes |
23.23 |
|
(e) U.S. standard conditions. In addition to the conditions in § 23.56, you must meet all of the following conditions:
(1) You may not export or re-export a plant (including its parts, products, or derivatives) under this certificate if the plant was removed from the wild or grown directly from a wild seed or spore, except for plants grown from exempt plant materials that qualify as artificially propagated.
(2) You may not export an Appendix–I species that was propagated for commercial purposes under this certificate, except for hybrids of one or more Appendix–I species or taxa that are not annotated to treat hybrids as Appendix–I specimens. (See § 23.47.)
(3) You may export a native plant under this certificate only when specifically approved for export and listed on the certificate, inventory sheet, or an approved species list.
(4) You may export a specimen under a higher-taxon name only if you identified the taxon in your application and we approved it on this certificate.
Credits
[79 FR 30425, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
§ 23.41 What are the requirements for a bred-in-captivity certificate?
(a) Purpose. Article VII(5) of the Treaty grants an exemption to wildlife that is bred in captivity when a Management Authority issues a certificate.
(b) U.S. and foreign general provisions. The following provisions apply to the issuance and acceptance of a certificate for Appendix–I, -II, or -III wildlife that was bred in captivity:
(1) The certificate and any subsequent re-export certificate must show the source code as “C” for bred in captivity.
(2) For an Appendix–I specimen that satisfies the requirements of this section, no CITES import permit is required.
(c) U.S. application form. Complete Form 3–200–24, 3–200–80, or 3–200–85 and submit it to the U.S. Management Authority.
(d) Criteria. The criteria in this paragraph (d) apply to the issuance and acceptance of U.S. and foreign certificates. When applying for a U.S. certificate, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
|
Appendix of the specimen |
||||
|
Criteria for a bred-in-captivity certificate |
I |
II |
III |
Section |
|
(1) The wildlife was bred in captivity. |
Yes |
Yes |
Yes |
23.63 |
|
(2) The wildlife specimen was bred for noncommercial purposes or is part of a traveling exhibition. |
Yes |
n/a |
n/a |
23.5 |
|
(3) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP. |
Yes |
Yes |
Yes |
23.23 |
|
(4) Live wildlife will be prepared and shipped so as to minimize risk of injury, damage to health, or cruel treatment of the specimen. |
Yes |
Yes |
Yes |
23.23 |
Credits
[79 FR 30425, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
§ 23.42 What are the requirements for a plant hybrid?
General provisions. Except as provided in § 23.92, the export, re-export, or import of a plant hybrid of a CITES species must be accompanied by a valid CITES document that shows the Appendix of the specimen as follows:
|
Question on a plant hybrid |
Answer and status of specimen |
|
(a) Is the specimen an artificially propagated hybrid of one or more Appendix-I species or taxa? |
(1) YES. Continue to paragraph (b) of this section. |
|
(2) NO. Continue to paragraph (c) of this section. |
|
|
(b) Is one or more of the Appendix-I species or taxa in paragraph (a) of this section annotated to treat hybrids as Appendix–I specimens? |
(1) YES. The hybrid is listed in Appendix I. |
|
(2) NO. The hybrid is listed in Appendix I, but may be granted a certificate for artificially propagated plants even if propagated for commercial purposes. |
|
|
(c) Is the specimen a hybrid that includes two or more CITES species or taxa in its lineage? |
(1) YES. Consider the specimen to be listed in the more restrictive Appendix, with Appendix I being the most restrictive and Appendix III the least. |
|
(2) NO. Continue to paragraph (d) of this section. |
|
|
(d) Is the specimen a hybrid that includes one CITES species or taxon in its lineage? |
(1) YES. Consider the specimen to be listed in the Appendix in which the species or taxon is listed in the CITES Appendices. |
|
(2) NO. The hybrid is not regulated by CITES. |
Credits
[79 FR 30425, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.43 What are the requirements for a wildlife hybrid?
(a) Definition. For the purposes of this section, recent lineage means the last four generations of a specimen's ancestry (direct line of descent).
(b) U.S. and foreign general provisions. Except as provided in paragraph (f) of this section, the import, export, or re-export of a hybrid CITES wildlife specimen must be accompanied by a valid CITES document.
(c) CITES documents. All CITES documents must show the wildlife hybrid listed in the following Appendix:
|
If at least one specimen in the recent lineage is listed in: |
Then the specimen is listed in: |
|
(1) Appendix I |
Appendix I |
|
(2) Appendix II, and an Appendix-I species is not included in the recent lineage |
Appendix II |
|
(3) Appendix III, and an Appendix-I or -II species is not included in the recent lineage |
Appendix III |
(d) U.S. application for wildlife hybrid. To apply for a CITES document, complete the appropriate form for the proposed activity (see §§ 23.18 through 23.20) and submit it to the U.S. Management Authority.
(e) Criteria. For export of a hybrid that contains a CITES species in its recent lineage, you must meet the requirements of § 23.36.
(f) Exempt wildlife hybrids. The following provisions apply to import, export, or re-export of exempt wildlife hybrids:
(1) A hybrid between a CITES species and a non–CITES species may be exempt from CITES document requirements if there are no purebred CITES species in the previous four generations of the specimen's ancestry (direct line of descent). Under this section, a hybrid between two CITES species is not exempt.
(2) For import, export, or re-export of an exempt wildlife hybrid without CITES documents, you must provide information at the time of import or export to clearly demonstrate that your specimen has no purebred CITES specimens in the previous four generations of its ancestry. If you are unable to clearly demonstrate this, you must obtain CITES documents. The information you provide must clearly identify the specimen and demonstrate its recent lineage. Such information may include, but is not limited to, the following:
(i) Records that identify the name and address of the breeder and identify the specimen by birth or hatch date and by sex, band number, microchip number, or other mark.
(ii) A certified pedigree issued by an internationally recognized association that contains scientific names of the animals in the specimen's recent lineage and clearly illustrates its genetic history. If the pedigree contains codes, you must provide a key or guide that explains the meaning of the codes.
(3) Although a CITES document is not required for an exempt wildlife hybrid, you must follow the clearance requirements for wildlife in part 14 of this subchapter, including the prior notification requirements for live wildlife.
Credits
[79 FR 30425, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
§ 23.44 What are the requirements to travel internationally with my personally owned live wildlife?
(a) Purpose. A Management Authority may use the exemption in Article VII(3) of the Treaty to issue a certificate of ownership that authorizes frequent cross-border movements of personally owned live wildlife for personal use.
(b) U.S. and foreign general provisions. The following provisions apply to the issuance and acceptance of a certificate of ownership for frequent international travel with live wildlife for personal use:
(1) The certificate must be obtained from the Management Authority in the country of the owner's primary residence.
(2) Parties should treat the certificate like a passport for import to and export or re-export from each country and should not collect the original certificate at the border.
(3) If offspring are born or an additional specimen is acquired while the owner is outside his or her country of primary residence, the owner must obtain the appropriate CITES document for the export or re-export of the wildlife, not a certificate of ownership, from the Management Authority of that country.
(4) Upon returning home, the owner may apply for a certificate of ownership for wildlife born or acquired overseas.
(c) U.S. application form. Complete Form 3–200–64 and submit it to the U.S. Management Authority.
(d) Criteria. The criteria in this paragraph (d) apply to the issuance and acceptance of U.S. and foreign certificates. When applying for a U.S. certificate, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
(1) The traveler owns the live wildlife and it will accompany the owner.
(2) The cross-border movement will be frequent and for personal use, including, but not limited to, companionship or use in a noncommercial competition such as falconry.
(3) To apply for a U.S. certificate, the owner resides in the United States.
(4) The wildlife was legally acquired (see § 23.60).
(5) The owner does not intend to sell, donate, or transfer the wildlife while traveling internationally.
(6) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP (see § 23.23).
(7) The Management Authority of the country of import has agreed to the cross-border movement.
(8) The wildlife is securely marked or uniquely identified in such a manner that the border official can verify that the specimen and CITES document correspond.
(9) The wildlife is transported and cared for in a way that minimizes risk of injury, damage to health, or cruel treatment of the specimen (see § 23.23).
(e) U.S. standard conditions. In addition to the conditions in § 23.56, all of the following conditions must be met:
(1) You must accompany the wildlife during any cross-border movement.
(2) You must transport the wildlife for personal use only.
(3) You must not sell, donate, or transfer the specimen while traveling internationally.
(4) You must present the certificate to the official for validation at each border crossing.
(5) If the certificate is lost, stolen, or accidentally destroyed, you must obtain a replacement certificate from the issuing Management Authority.
(6) If you no longer own the live wildlife, you must immediately return the original document to the issuing Management Authority and report on the disposition of the wildlife, such as death, sale, or transfer.
(7) You must return the wildlife to the United States before the certificate expires.
Credits
[79 FR 30425, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
§ 23.45 What are the requirements for a pre-Convention specimen?
(a) Purpose. Article VII(2) of the Treaty exempts a pre-Convention specimen from standard permitting requirements in Articles III, IV, and V of the Treaty when the exporting or re-exporting country is satisfied that the specimen was acquired before the provisions of CITES applied to it and issues a CITES document to that effect.
(b) U.S. and foreign general provisions. The following general provisions apply to the issuance and acceptance of pre-Convention documents:
(1) Trade in a specimen under the pre-Convention exemption is allowed only if the importing country will accept a pre-Convention certificate.
(2) The pre-Convention date is the date the species was first listed under CITES regardless of whether the species has subsequently been transferred from one Appendix to another.
(3) For a pre-Convention Appendix-I specimen, no CITES import permit is required.
(4) The pre-Convention exemption does not apply to offspring or cell lines of any wildlife or plant born or propagated after the date the species was first listed under CITES.
(c) U.S. application form. Complete Form 3-200-23 (wildlife) or Form 3-200-32 (plants) and submit it to the U.S. Management Authority.
(d) Criteria. The criteria in this paragraph (d) apply to the issuance and acceptance of U.S. and foreign certificates. When applying for a U.S. certificate, you must provide sufficient information for us to find that the specimen meets all of the following criteria:
(1) The specimen was removed from the wild or born or propagated in a controlled environment before the date CITES first applied to it, or is a product (including a manufactured item) or derivative made from such specimen.
(2) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP (see § 23.23).
(3) Live wildlife or plants will be prepared and shipped so as to minimize risk of injury, damage to health, or cruel treatment of the specimen.
(4) For the re-export of a pre-Convention specimen previously imported under a CITES document, the wildlife or plant was legally imported.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.46 What are the requirements for registering a commercial breeding operation for Appendix–I wildlife and commercially exporting specimens?
(a) Purpose. Article VII(4) of the Treaty provides that Appendix–I specimens that are bred in captivity for commercial purposes shall be deemed to be listed in Appendix II. This means that an Appendix–I specimen originating from a commercial breeding operation that is registered with the CITES Secretariat may be traded under an export permit or re-export certificate based on Appendix–II criteria. The specimen is still listed in Appendix I and is not eligible for any exemption granted to an Appendix–II species or taxon, including any exemption granted by an annotation (see § 23.92).
(b) U.S. and foreign general provisions. The following provisions apply to the registration of U.S. and foreign commercial breeding operations for Appendix–I wildlife:
(1) If the Management Authority is satisfied that the operation in its country meets the conditions for registration in paragraph (d) of this section, it will send the request to register a breeding operation to the Secretariat.
(2) The Secretariat will verify that the application is complete and notify the Parties of the request.
(3) If any Party objects to or expresses concern about the registration within 90 days from the date of the Secretariat's notification, the Secretariat will refer the application to the Animals Committee. The Committee has 60 days to respond to objections. The Secretariat will provide the recommendations of the Committee to the Management Authority of the Party that submitted the application and the Party that objected to the registration, and will allow a further 30 days for resolution of the identified problems.
(4) If the objection is not withdrawn or the identified problems are not resolved within the 30–day period, the Secretariat will submit the application to the Standing Committee at its next regular meeting. The Standing Committee will determine whether the objection is justified and decide whether to accept the application.
(5) When the Secretariat is satisfied that the operation meets the registration requirements, it will include the operation in its register.
(6) Operations are assigned an identification number and listed in the official register. Registration is not final until the Secretariat notifies all Parties.
(7) If a Party believes that a registered operation does not meet the bred-in-captivity requirements, it may, after consultation with the Secretariat and the Party concerned, propose to the Standing Committee that the operation be deleted from the register. At its following meeting, the Standing Committee will consider the concerns raised by the objecting Party, and any comments from the registering Party and the Secretariat, and determine whether the operation should be deleted from the register. Once an operation has been deleted, it must re-apply and meet the registration requirements to be reinstated.
(8) The Management Authority, in collaboration with the Scientific Authority, of a country where any registered operation is located must monitor the operation to ensure that it continues to meet the registration requirements. In the United States, we will monitor registered operations, in part, by requiring each operation to apply for renewal and demonstrate that it continues to qualify for registration at least once every 5 years. (See paragraphs (e)(4) and (f) of this section.) The Management Authority will advise the Secretariat of any major change in the nature of the operation or in the types of products being produced for export.
(9) A Party may unilaterally request the removal of a registered operation within its jurisdiction by notifying the Secretariat.
(10) An Appendix–I specimen may not be imported for purposes of establishing or augmenting a commercial breeding operation, unless the specimen is pre–Convention (see § 23.45) or was bred in captivity (see § 23.63).
(c) U.S. application to register. Complete Form 3–200–65 and submit it to the U.S. Management Authority.
(d) Criteria. The criteria in this paragraph (d) apply to the registration of U.S. and foreign commercial breeding operations for Appendix–I wildlife. For your breeding operation to be registered in the United States, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
|
Criteria for registering a commercial breeding operation for Appendix-I wildlife |
Section |
|
(1) The operation breeds wildlife for commercial purposes. |
23.5 |
|
(2) The parental stock was legally acquired. |
23.60 |
|
(3) The wildlife meets bred-in-captivity criteria. |
23.63 |
|
(4) Where the establishment of a breeding operation involves the removal of animals from the wild (allowable only under exceptional circumstances and only for native species), the operation must demonstrate to the satisfaction of the Management Authority, on advice of the Scientific Authority and of the Secretariat, that the removal is or was not detrimental to the conservation of the species. |
- |
|
(5) The potential escape of specimens or pathogens from the facility does not pose a risk to the ecosystem and native species. |
- |
|
(6) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP. |
23.23 |
|
(7) The breeding operation will make a continuing, meaningful contribution to the conservation of the species according to the conservation needs of the species. |
- |
|
(8) The operation will be carried out at all stages in a humane (non-cruel) manner. |
- |
(e) Standard conditions of the registration. In addition to the conditions in § 23.56, you must meet all of the following conditions:
(1) You must uniquely mark all specimens from the breeding operation in the manner proposed at the time of registration. Birds may be marked with closed bands, although other methods may be used.
(2) You may not import Appendix–I specimens for primarily commercial purposes (such as to establish a commercial captive-breeding operation) except from breeding operations registered for that species.
(3) You must allow our agents to enter the premises at any reasonable hour to inspect wildlife held or to inspect, audit, or copy applicable records.
(4) Registrations will be valid for a period not to exceed 5 years. Registrants who wish to remain registered must request renewal before the end of the period of validity of the registration.
(f) U.S. application to renew a registration. Requests for renewal of a registration should be submitted at least 3 months before the registration expires. Complete Form 3–200–65 and submit it to the U.S. Management Authority.
(g) Criteria for renewal of U.S. registrations. To renew your registration, you must provide sufficient information for us to find that your proposed activity continues to meet all of the criteria in paragraph (d) of this section.
(h) U.S. and foreign general provisions for export of specimens that originated in a registered breeding operation. The following provisions apply to the issuance and acceptance of export permits for Appendix–I specimens bred at an operation registered with the CITES Secretariat:
(1) An export permit may be issued to the registered operation or to persons who have purchased a specimen that originated at the registered operation if the specimen has the unique mark applied by the operation. If a microchip is used, we may, if necessary, ask the importer, exporter, or re-exporter to have equipment on hand to read the microchip at the time of import, export, or re-export.
(2) The export permit, and any subsequent re-export certificate, must show the specimen as listed in Appendix I and the source code as “D,” and give the identification number of the registered breeding operation where the specimen originated.
(3) No CITES import permit is required for a qualifying specimen.
(i) U.S. application form. Complete the appropriate form (see § 23.36) and submit it to the U.S. Management Authority.
(j) Criteria. The criteria in this paragraph (h) apply to the issuance and acceptance of U.S. and foreign export permits. When applying for a U.S. permit, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
|
Criteria for an export permit |
Section |
|
(1) The specimen was bred at a commercial operation for Appendix-I wildlife that is registered with the CITES Secretariat. |
23.46 |
|
(2) The proposed export would not be detrimental to the survival of the species. |
23.61 |
|
(3) Live wildlife will be prepared and shipped so as to minimize risk of injury, damage to health, or cruel treatment of the specimen. |
23.23 |
Credits
[79 FR 30425, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.47 What are the requirements for export of an Appendix–I plant artificially propagated for commercial purposes?
(a) Purpose. Article VII(4) of the Treaty provides that Appendix–I plants artificially propagated for commercial purposes shall be deemed to be listed in Appendix II. This means that an Appendix–I specimen originating from a commercial nursery that is registered with the CITES Secretariat or that meets the requirements of this section may be traded under an export permit or re-export certificate based on Appendix–II criteria. The specimen is still listed in Appendix I and is not eligible for any exemption granted to an Appendix–II species or taxon, including any exemption granted by an annotation. This section does not apply to hybrids of one or more Appendix–I species or taxa that are not annotated to treat hybrids as Appendix–I specimens (see § 23.40).
(b) U.S. and foreign general provisions. The following provisions apply to the issuance and acceptance of export permits for Appendix–I specimens artificially propagated for commercial purposes:
(1) An Appendix–I specimen may not be imported for purposes of establishing or augmenting a nursery or commercial propagating operation, unless the specimen is pre–Convention (see § 23.45) or was propagated at a nursery that is registered with the CITES Secretariat or a commercial propagating operation that qualifies under paragraph (d) of this section, and the CITES document indicates the source code as “D.”
(2) An export permit may be issued to a CITES–registered nursery, to a commercial propagating operation that qualifies under paragraph (d) of this section, or to persons who have acquired a specimen that originated at such a nursery or operation. No CITES import permit is required for a qualifying specimen.
(3) The export permit, and any subsequent re-export certificate, must show the specimen as listed in Appendix I and the source code as “D,” and if from a nursery registered with the Secretariat, give the identification number of the registered nursery where the specimen originated.
(c) U.S. application form. Complete Form 3–200–33 or Form 3–200–74 (for additional single-use permits under a master file or an annual export program file). Complete Form 3–200–32 for one-time export. Submit the completed form to the U.S. Management Authority.
(d) Criteria. The criteria in this paragraph (d) apply to the issuance and acceptance of U.S. and foreign export permits. When applying for a U.S. permit, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
|
Criteria for an export permit |
Section |
|
(1) The specimen was propagated for commercial purposes. |
23.5 |
|
(2) The parental stock was legally acquired. |
23.60 |
|
(3) The proposed export would not be detrimental to the survival of the species. |
23.61 |
|
(4) The plant was artificially propagated. |
23.64 |
|
(5) The scientific name of the species is the standard nomenclature in the CITES Appendices or the references adopted by the CoP. |
23.23 |
|
(6) The live plant will be prepared and shipped so as to minimize risk of injury, damage to health, or cruel treatment of the specimen. |
23.23 |
(e) Nursery registration. [Reserved]
Credits
[79 FR 30426, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.48 What are the requirements for a registered scientific institution?
(a) Purpose. Article VII(6) of the Treaty grants an exemption that allows international trade in certain specimens for noncommercial loan, donation, or exchange between registered scientific institutions.
(b) U.S. and foreign general provisions. The following provisions apply to the registration of scientific institutions and acceptance of shipments from registered scientific institutions:
(1) The receiving and sending scientific institutions must be registered with the Management Authority in their country. Scientists who wish to use this exemption must be affiliated with a registered scientific institution.
(i) When a Management Authority is satisfied that a scientific institution has met the criteria for registration, it will assign the institution a five-character code consisting of the ISO country code and a unique three-digit number. In the case of a non-Party, the Secretariat will ensure that the institution meets the standards and assign it a unique code.
(ii) The Management Authority must communicate the name, address, and assigned code to the Secretariat, which maintains a register of scientific institutions and provides that information to all Parties.
(2) A registered scientific institution does not need separate CITES documents for the noncommercial loan, donation, or exchange of preserved, frozen, dried, or embedded museum specimens, herbarium specimens, or live plant material with another registered institution. The shipment must have an external label that contains information specified in paragraph (e)(5) of this section.
(c) U.S. application to register as a scientific institution. To register, complete Form 3-200-39 and submit it to the U.S. Management Authority.
(d) Criteria. The criteria in this paragraph (d) apply to the registration of U.S. and foreign institutions for scientific exchange. To be issued a certificate of scientific exchange as a registered U.S. scientific institution, you must provide sufficient information for us to find that your institution meets all of the following criteria:
(1) Collections of wildlife or plant specimens are permanently housed and professionally curated, and corresponding records are kept.
(2) Specimens are accessible to all qualified users, including those from other institutions.
(3) Specimens are properly accessioned in a permanent catalog.
(4) Records are permanently maintained for loans and transfers to and from other institutions.
(5) Specimens are acquired primarily for research that is to be reported in scientific publications, and CITES specimens are not used for commercial purposes or as decorations.
(6) Collections are prepared and arranged in a way that ensures their accessibility to researchers.
(7) Specimen labels, permanent catalogs, and other records are accurate.
(8) Specimens are legally acquired and lawfully possessed under a country's wildlife and plant laws.
(9) Appendix-I specimens are permanently and centrally housed under the direct control of the institution.
(e) U.S. standard conditions. In addition to the conditions in § 23.56, any activity conducted under a certificate of scientific exchange must meet all of the following conditions:
(1) Both scientific institutions involved in the exchange must be registered by the applicable Management Authorities (or the Secretariat in the case of a non-Party), and be included in the Secretariat's register of scientific institutions.
(2) An institution may send and receive only preserved, frozen, dried, or embedded museum specimens, herbarium specimens, or live plant materials that have been permanently and accurately recorded by one of the institutions involved in the exchange and that are traded as a noncommercial loan, donation, or exchange.
(3) An institution may use specimens acquired under a certificate of scientific exchange and their offspring only for scientific research or educational display at a scientific institution and may not use specimens for commercial purposes.
(4) The institution must keep records to show that the specimens were legally acquired.
(5) A customs declaration label must be affixed to the outside of each shipping container or package that contains all of the following:
(i) The acronym “CITES.”
(ii) A description of the contents (such as “herbarium specimens”).
(iii) The names and addresses of the sending and receiving registered institutions.
(iv) The signature of a responsible officer of the sending registered scientific institution.
(v) The scientific institution codes of both registered scientific institutions involved in the loan, donation, or exchange.
(6) A registered institution may destroy samples during analysis, provided that a portion of the sample is maintained and permanently recorded at a registered scientific institution for future scientific reference.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.49 What are the requirements for an exhibition traveling internationally?
(a) Purpose. Article VII(7) of the Treaty grants an exemption for specimens that qualify as bred in captivity, artificially propagated, or pre-Convention and are part of a traveling exhibition.
(b) U.S. and foreign general provisions. The following general provisions apply to the issuance and acceptance of a certificate for live wildlife and plants, or their parts, products, or derivatives in an exhibition that travels internationally:
(1) The Management Authority in the country of the exhibitor's primary place of business must have determined that the specimens are bred in captivity, artificially propagated, or pre-Convention and issued a traveling-exhibition certificate.
(2) The certificate must indicate that the wildlife or plant is part of a traveling exhibition.
(3) A separate certificate must be issued for each live wildlife specimen; a CITES document may be issued for more than one specimen for a traveling exhibition of live plants and dead parts, products, or derivatives of wildlife and plants.
(4) The certificate is not transferable.
(5) Parties should treat the certificate like a passport for import and export or re-export from each country, and should not collect the original certificate at the border.
(6) Parties should check specimens closely to determine that each specimen matches the certificate and ensure that each live specimen is being transported and cared for in a manner that minimizes the risk of injury, damage to health, or cruel treatment of the specimen.
(7) If offspring are born or a new specimen is acquired while the traveling exhibition is in another country, the exhibitor must obtain the appropriate CITES document for the export or re-export of the specimen from the Management Authority of that country.
(8) Upon returning home, the exhibitor may apply for a traveling-exhibition certificate for wildlife born overseas or for wildlife or plants acquired overseas.
(c) U.S. application form. Complete Form 3-200-30 for wildlife and Form 3-200-32 for plants, and submit it to the U.S. Management Authority.
(d) Criteria. The criteria in this paragraph (d) apply to the issuance and acceptance of U.S. and foreign certificates. When applying for a U.S. certificate, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
(1) The traveling exhibition makes multiple cross-border movements, and will return to the country in which the exhibition is based before the certificate expires.
(2) The cross-border movement must be for exhibition, and not for breeding, propagating, or activities other than exhibition.
(3) The traveling exhibition is based in the country that issued the certificate.
(4) The specimen meets the criteria for a bred-in-captivity certificate, certificate for artificially propagated plants, or pre-Convention certificate.
(5) The exhibitor does not intend to sell or otherwise transfer the wildlife or plant while traveling internationally.
(6) The wildlife or plant is securely marked or identified in such a way that border officials can verify that the certificate and specimen correspond. If a microchip is used, we may, if necessary, ask the importer, exporter, or re-exporter to have equipment on hand to read the microchip at the time of import, export, or re-export.
(e) U.S. standard conditions. In addition to the conditions in § 23.56, you must meet all of the following conditions:
(1) The certificate may be used by you, and you must not transfer or assign it to another person or traveling exhibition.
(2) You must transport the specimen internationally only for exhibition, not for breeding, propagating, or activities other than exhibition.
(3) You must present the certificate to the official for validation at each border crossing.
(4)For live plants, the quantity of plants must be reasonable for the purpose of the traveling exhibition.
(5) You must not sell or otherwise transfer the specimen, or any offspring born to such specimen, while traveling internationally.
(6) If the certificate is lost, stolen, or accidentally destroyed, you may obtain a replacement certificate only from the U.S. Management Authority.
(7) If you no longer own the wildlife or plants, or no longer plan to travel as a traveling exhibition, the original certificate must be immediately returned to the U.S. Management Authority.
(8) You must return the traveling exhibition to the United States before the certificate expires.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.50 What are the requirements for a sample collection covered by an ATA carnet?
(a) Purpose. Article VII(1) of the Treaty allows for the transit of specimens through or within a Party country while the specimens remain under customs control.
(b) Definition. For purposes of this section, sample collection means a set of legally acquired parts, products, or derivatives of Appendix-II or -III species, or Appendix-I species bred in captivity or artificially propagated for commercial purposes, that will:
(1) Cross international borders only for temporary exhibition or display purposes and return to the originating country.
(2) Be accompanied by a valid ATA carnet and remain under customs control.
(3) Not be sold or otherwise transferred while traveling internationally.
(c) U.S. and foreign general provisions. The following general provisions apply to the issuance and acceptance of a CITES document for the movement of sample collections:
(1) The Management Authority in the country where the sample collection originated must issue a CITES document that:
(i) Clearly specifies that the document was issued for a “sample collection.”
(ii) Includes the condition in block 5, or an equivalent place, of the document that it is valid only if the shipment is accompanied by a valid ATA carnet and that the specimens must not be sold, donated, or otherwise transferred while outside the originating country.
(2) The number of the accompanying ATA carnet must be recorded on the CITES document, and if this number is not recorded by the Management Authority, it must be entered by a customs or other CITES enforcement official responsible for the original endorsement of the CITES document.
(3) The name and address of the exporter or re-exporter and importer must be identical, and the names of the countries to be visited must be indicated in block 5 or an equivalent place.
(4) The date of validity must not be later than that of the ATA carnet and the period of validity must not exceed 6 months from the date of issuance.
(5) At each border crossing, Parties must verify the presence of the CITES document, but allow it to remain with the shipment, and ensure that the ATA carnet is properly endorsed with an authorized stamp and signature by a customs official.
(6) The exporter or re-exporter must return the sample collection to the originating country prior to the expiration of the CITES document.
(7) Parties should check the CITES document and sample collection closely at the time of first export or re-export and upon its return to ensure that the contents of the sample collection have not been changed.
(8) For import into and export or re-export from the United States, the shipment must comply with the requirements for wildlife in part 14 of this subchapter and for plants in part 24 of this subchapter and 7 CFR parts 319, 352, and 355.
(d) U.S. application form. Complete Form 3-200-29 for wildlife and Form 3-200-32 for plants, and submit it to the U.S. Management Authority.
(e) Criteria. The criteria in this paragraph (e) apply to the issuance and acceptance of U.S. and foreign documents. When applying for a U.S. document, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
(1) The specimens meet the definition of a sample collection as provided in paragraph (b) of this section.
(2) The wildlife or plant specimens must be securely marked or identified in such a way that border officials can verify that the CITES document, ATA carnet, and specimens correspond.
(f) U.S. standard conditions. In addition to the conditions in § 23.56, you must meet all of the following conditions:
(1) You must transport the sample collection only for temporary exhibition or display purposes.
(2) You must not transfer or assign the CITES document to another person.
(3) You must not sell, donate, or transfer specimens while traveling internationally.
(4) You must present the CITES document and the ATA carnet to the official for validation at each border crossing.
(5) You must return the sample collection to the United States prior to the expiration of the CITES document.
(6) If the CITES document is lost, stolen, or accidentally destroyed, you may obtain a replacement certificate only from the U.S. Management Authority.
(7) If you no longer own the sample collection, or no longer plan to travel with the sample collection, you must immediately return the original document to the U.S. Management Authority.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.51 What are the requirements for issuing a partially completed CITES document?
(a) Purpose. Under Article VIII(3), Parties are to ensure that CITES specimens are traded with a minimum of delay.
(b) U.S. and foreign general provisions. The following provisions apply to the issuance and acceptance of partially completed CITES documents.
(1) A Management Authority may issue partially completed CITES documents only when:
(i) The permitted trade will have a negligible impact or no impact on the conservation of the species.
(ii) All provisions of CITES have been met.
(iii) The specimens are one of the following:
(A) Biological samples.
(B) Pre-Convention specimens.
(C) Specimens that qualify as bred in captivity or artificially propagated.
(D) Appendix-I specimens from registered commercial breeding operations.
(E) Appendix-I plants artificially propagated for commercial purposes.
(F) Other specimens that the Management Authority determines qualify for partially completed documents.
(2) A Management Authority may register applicants for species that may be traded under partially completed documents.
(3) Partially completed CITES documents require the permit holder to:
(i) Enter specific information on the CITES document or its annex as conditioned on the face of the CITES document.
(ii) Enter scientific names on the CITES document only if the Management Authority included an inventory of approved species on the face of the CITES document or an attached annex.
(iii) Sign the CITES document, which acts as a certification that the information entered is true and accurate.
(4) CITES documents issued for biological samples may be validated at the time of issuance provided that upon export the container is labeled with the CITES document number and indicates it contains CITES biological samples.
(c) U.S. application form. Complete the appropriate form for the proposed activity (see §§ 23.18 through 23.20) and submit it to the U.S. Management Authority.
(d) Criteria. The criteria in this paragraph (d) apply to the issuance and acceptance of U.S. and foreign CITES documents. When applying for a U.S. CITES document, you must provide sufficient information for us to find that your proposed activity meets the criteria in subpart C for the appropriate CITES document and the following criteria:
(1) The use of partially completed documents benefits both the permit holder and the issuing Management Authority.
(2) The proposed activity will have a negligible impact or no impact upon the conservation of the species.
(e) U.S. standard conditions. In addition to the conditions in § 23.56 and any standard conditions in this part that apply to the specific CITES document, the following conditions must be met:
(1) You must enter the information specified in block 5, either on the face of the CITES document or in an annex to the document.
(2) You may not alter or enter any information on the face of the CITES document or in an annex to the document that is not authorized in block 5 or an equivalent place.
(3) If you are authorized to enter a scientific name, it must be for a species authorized in block 5 or an equivalent place, or in an attached annex of the CITES document.
(4) You must sign the CITES document to certify that all information entered by you is true and correct.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.52 What are the requirements for replacing a lost, damaged, stolen, or accidentally destroyed CITES document?
(a) Purpose. A Management Authority may issue a duplicate document, either a copy of the original or a re-issued original, when a CITES document has been lost, damaged, stolen, or accidentally destroyed. These provisions do not apply to a document that has expired or that requires amendment. To renew a U.S. CITES document, see part 13 of this subchapter. To amend a U.S. CITES document, see part 13 of this subchapter if the activity has not yet occurred or, if the activity has already occurred, see § 23.53 of this part.
(b) U.S. and foreign general provisions. The following provisions apply to the issuance and acceptance of a replacement CITES document:
(1) The permittee must notify the issuing Management Authority that the document was lost, damaged, stolen, or accidentally destroyed.
(2) The issuing Management Authority must be satisfied that the CITES document was lost, damaged, stolen, or accidentally destroyed.
(3) The issuing Management Authority should immediately inform the Management Authority in the country of destination and, for commercial shipments, the Secretariat.
(4) If the replacement CITES document is a copy, it must indicate that it is a “replacement” and a “true copy of the original,” contain a new dated original signature of a person authorized to sign CITES documents for the issuing Management Authority, and give the reason for replacement.
(5) If the replacement CITES document is a newly issued original document, it must indicate that it is a “replacement,” include the number and date of issuance of the document being replaced, and give the reason for replacement.
(6) In the United States, you may not use an original single-use CITES document issued under a CITES master file or CITES annual program as a replacement document for a shipment that has already left the country.
(c) U.S. application procedures. To apply for a replacement CITES document, you must do all of the following:
(1) Complete application Form 3–200–66 and submit it to the U.S. Management Authority.
(2) Consult the list to find the types of information you need to provide (more than one circumstance may apply to you):
|
If |
Then |
|
|
(i) The shipment has already occurred |
Provide copies of: |
|
|
(A) Any correspondence you have had with the shipper or importing country's Management Authority concerning the shipment. |
||
|
(B) For wildlife, the validated CITES document and cleared Declaration for Importation or Exportation of Fish or Wildlife (Form 3-177). |
||
|
(C) For plants, the validated CITES document. |
||
|
(ii) The original CITES document no longer exists |
Submit a signed, dated, and notarized statement that: |
|
|
(A) Provides the CITES document number and describes the circumstances that resulted in the loss or destruction of the original CITES document. |
||
|
(B) States whether the shipment has already occurred. |
||
|
(C) Requests a replacement U.S. CITES document. |
||
|
(iii) An original CITES document exists but has been damaged |
Submit the original damaged CITES document and a signed, dated, and notarized statement that: |
|
|
(A) Describes the circumstances that resulted in the CITES document being damaged. |
||
|
(B) States whether the shipment has already occurred. |
||
|
(C) Requests a replacement U.S. CITES document. |
||
(d) Criteria. The criteria in this paragraph (d) apply to the issuance and acceptance of U.S. and foreign documents.
(1) When applying for a U.S. replacement document, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
(i) The circumstances for the lost, damaged, stolen, or accidentally destroyed CITES document are reasonable.
(ii) If the shipment has already been made, the wildlife or plant was legally exported or re-exported, and the Management Authority of the importing country has indicated it will accept the replacement CITES document.
(iii) The specimens were presented to the appropriate official for inspection at the time of import and a request for a replacement CITES document was made at that time.
(2) For acceptance of foreign CITES replacement documents in the United States, you must provide sufficient information for us to find that your proposed activity meets all of the following criteria:
(i) The specimens were presented to the appropriate official for inspection at the time of import and a request for a replacement CITES document was made at that time.
(ii) The importer or the importer's agent submitted a signed, dated, and notarized statement at the time of import that describes the circumstances that resulted in the CITES document being lost, damaged, stolen, or accidentally destroyed.
(iii) The importer or the importer's agent provided a copy of the original lost, stolen, or accidentally destroyed document at the time of import showing that the document met the requirements in §§ 23.23, 23.24, and 23.25.
(e) U.S. standard conditions. In addition to the conditions in § 23.56, the following conditions apply:
(1) If the original CITES document is found, you must return it to the U.S. Management Authority.
(2) A CITES document issued for a shipment that has already occurred does not require validation.
(f) Validation. For an export or re-export that has not left the United States, follow the procedures in § 23.27. If the shipment has left the United States and is in a foreign country, submit the unvalidated replacement CITES document to the appropriate foreign authorities. We will not validate the replacement CITES document for a shipment that has already been shipped to a foreign country. We do not require validation on replacement documents issued by foreign Management Authorities.
Credits
[79 FR 30426, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.53 What are the requirements for obtaining a retrospective CITES document?
(a) Retrospective CITES documents may be issued and accepted in certain limited situations after an export or re-export has occurred, but before the shipment is cleared for import. When specific conditions are met, a retrospective CITES document may be issued to authorize trade that has taken place without a CITES document or to correct certain technical errors in a CITES document after the authorized activity has occurred.
(b) U.S. and foreign general provisions. The following provisions apply to the issuance and acceptance of a retrospective CITES document:
(1) A retrospective document may not be issued for Appendix–I specimens except for certain specimens for personal use as specified in paragraph (d)(7) of this section.
(2) The exporter or re-exporter must notify the Management Authority in the exporting or re-exporting country of the irregularities that have occurred.
(3) A retrospective document may be one of the following:
(i) An amended CITES document where it can be shown that the issuing Management Authority made a technical error that was not prompted by the applicant.
(ii) A newly issued CITES document where it can be shown that the applicant was misinformed by CITES officials or the circumstances in (d)(7) of this section apply and a shipment has occurred without a document.
(4) Retrospective documents can only be issued after consultation between the Management Authorities in both the exporting or re-exporting country and the importing country, including a thorough investigation of circumstances and agreement between them that criteria in paragraph (d) of this section have been met.
(5) The issuing Management Authority must provide all of the following information on any retrospective CITES document:
(i) A statement that it was issued retrospectively.
(ii) A statement specifying the reason for the issuance.
(iii) In the case of a document issued for personal use, a condition restricting sale of the specimen within 6 months following the import of the specimen.
(6) The issuing Management Authority must send a copy of the retrospective CITES document to the Secretariat.
(7) In general, except when the exporter or re-exporter and importer have demonstrated they were not responsible for the irregularities, any person who has been issued a CITES document in the past will not be eligible to receive a retrospective document.
(8) In the United States, you may not use a U.S. CITES document issued under a CITES master file or CITES annual program as a retrospective CITES document.
(c) U.S. application. Complete application Form 3–200–58 and submit it to the U.S. Management Authority. In addition, submit one of the following:
(1) For a shipment that occurred under a document containing a technical error, the faulty CITES document.
(2) For a shipment that occurred without a CITES document, a completed application form for the type of activity you conducted (see §§ 23.18 through 23.20).
(d) Criteria. The criteria in this paragraph (d) apply to the issuance and acceptance of U.S. and foreign documents. When applying for a U.S. document, you must provide sufficient information for us to find that your activity meets all of the following criteria:
(1) The specimens were exported or re-exported without a CITES document or with a CITES document that contained technical errors as provided in paragraph (d)(6)(ii) of this section.
(2) The specimens were presented to the appropriate official for inspection at the time of import and a request for a retrospective CITES document was made at that time.
(3) The export or re-export and import of the specimens was otherwise in compliance with CITES and the relevant national legislation of the countries involved.
(4) The importing Management Authority has agreed to accept the retrospectively issued CITES document.
(5) The specimens must be Appendix–II or -III wildlife or plants, except as provided in paragraph (d)(7) of this section.
(6) Except as provided in paragraph (d)(7) of this section, the exporter or re-exporter and importer were not responsible for the irregularities that occurred and have demonstrated one of the following:
(i) The Management Authority or officials designated to clear CITES shipments misinformed the exporter or re-exporter or the importer about the CITES requirements. In the United States, this would be an employee of the FWS (for any species) or APHIS or CBP (for plants).
(ii) The Management Authority made a technical error when issuing the CITES document that was not prompted by information provided by the applicant.
(7) In the case of specimens for personal use, you must either show that you qualify under paragraph (d)(6) of this section, or that a genuine error was made and that there was no attempt to deceive. The following specimens for personal use may qualify for issuance of a retrospective document:
(i) Personal or household effects as defined in § 23.5.
(ii) Live Appendix–II or -III specimens or live pre–Convention Appendix–I specimens that you own for your personal use, accompanied you, and number no more than two.
(iii) Parts, products, or derivatives of an Appendix–I species that qualify as pre–Convention when the following conditions are met:
(A) You own and possess the specimen for personal use.
(B) You either wore the specimen as clothing or an accessory or took it as part of your personal baggage, which was carried by you or checked as baggage on the same plane, boat, car, or train as you.
(C) The quantity is reasonably necessary or appropriate for the nature of your trip or stay.
(e) U.S. standard conditions. In addition to the conditions in § 23.56, the following condition applies: A CITES document issued for a shipment that has already occurred does not require validation.
(f) Validation. Submit the original unvalidated retrospective CITES document to the appropriate foreign authority. We will not validate the retrospective CITES document for a shipment that has already been shipped to a foreign country, and we do not require validation on retrospective documents issued by foreign Management Authorities.
Credits
[79 FR 30426, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.54 How long is a U.S. or foreign CITES document valid?
(a) Purpose. Article VI(2) of the Treaty sets the time period within which an export permit is valid. Validity periods for other CITES documents are prescribed in this section.
(b) Period of validity. CITES documents are valid only if presented for import or introduction from the sea within the period of validity (before midnight on the expiration date) noted on the face of the document.
(1) An export permit and re-export certificate will be valid for no longer than 6 months from the issuance date.
(2) An import permit, introduction-from-the-sea certificate, and certificate of origin will be valid for no longer than 12 months from the issuance date.
(3) A traveling-exhibition certificate and certificate of ownership will be valid for no longer than 3 years from the issuance date.
(4) Other CITES documents will state the period of their validity, but no U.S. CITES document will be valid for longer than 3 years from the issuance date.
(c) Extension of validity. The validity of a CITES document may not be extended beyond the expiration date on the face of the document, except under limited circumstances for certain timber species as outlined in § 23.73.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.55 How may I use a CITES specimen after import into the United States?
In addition to the provisions in § 23.3, you may only use CITES specimens after import into the United States for the following purposes:
|
If the species is listed in |
Allowed use within the United States |
|
|
(a) Appendix I, except for specimens imported with a CITES exemption document listed in paragraph (d) of this section. |
The specimen may be used only for noncommercial purposes (see § 23.5). |
Exception: |
|
(b) Appendix II with an annotation for noncommercial purposes where other specimens of that species are treated as if listed in Appendix I. |
If the specimen was lawfully imported, with no restrictions on its use after import, before the species was listed as described in paragraphs (a), (b), or (c) of this section, you may continue to use the specimen as indicated for paragraphs (d), (e) and (f) of this section provided you can clearly demonstrate (using written records or other documentary evidence) that your specimen was imported prior to the CITES listing, with no restrictions on its use after import. If you are unable to clearly demonstrate that this exception applies, the specimen may be used only for noncommercial purposes. |
|
|
(c) Appendix II without an annotation for noncommercial purposes, or Appendix III, and threatened under the ESA, except as provided in a special rule in §§ 17.40 through 17.48 or under a permit granted under §§ 17.32 or 17.52 |
||
|
(d) Appendix I, and imported with a CITES exemption document as follows: |
The specimen may be used for any lawful purpose, except if the regulations in this part or other parts of this subchapter or a permit condition allowed the import only for noncommercial purposes, then the import and subsequent use must be only for noncommercial purposes. |
|
|
(1) U.S-issued certificate for personally owned wildlife. |
||
|
(2) Pre-Convention certificate. |
||
|
(3) Export permit or re-export certificate for wildlife from a registered commercial breeding operation. |
||
|
(4) Export permit or re-export certificate for a plant from a registered nursery or under a permit with a source code of “D.” |
||
|
(5) Certificate for artificially propagated plants with a source code of “A” for artificially propagated hybrid specimens derived from one or more unannotated Appendix-I species or other taxa. |
||
|
(6) U.S.-issued traveling-exhibition certificate. |
||
|
(e) Appendix II, other than those in paragraphs (b) and (c) of this section. |
||
|
(f) Appendix III, other than those in paragraph (c) of this section. |
Credits
[79 FR 30426, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.56 What U.S. CITES document conditions do I need to follow?
(a) General conditions. The following general conditions apply to all U.S. CITES documents:
(1) You must comply with the provisions of part 13 of this subchapter as conditions of the document, as well as other applicable regulations in this subchapter, including, but not limited to, any that require permits. You must comply with all applicable local, State, Federal, tribal, and foreign wildlife or plant conservation laws.
(2) For export and re-export of live wildlife and plants, transport conditions must comply with the International Air Transport Association Live Animals Regulations (for animals) or the International Air Transport Association Perishable Cargo Regulations (for plants) (incorporated by reference, see § 23.9).
(3) You must return the original CITES document to the issuing office if you do not use it, it expires, or you request renewal or amendment.
(4) When appropriate, a Management Authority may require that you identify Appendix–II and -III wildlife or plants with a mark. All live Appendix–I wildlife must be securely marked or uniquely identified. Such mark or identification must be made in a way that the border official can verify that the specimen and CITES document correspond. If a microchip is used, we may, if necessary, ask the importer, exporter, or re-exporter to have equipment on hand to read the microchip at the time of import, export, or re-export.
(b) Standard conditions. You must comply with the standard conditions provided in this part for specific types of CITES documents.
(c) Special conditions. We may place special conditions on a CITES document based on the needs of the species or the proposed activity. You must comply with any special conditions contained in or attached to a CITES document.
Credits
[79 FR 30427, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
Subpart D. Factors Considered in Making Certain Findings
§ 23.60 What factors are considered in making a legal acquisition finding?
(a) Purpose. Articles III, IV, and V of the Treaty require a Management Authority to make a legal acquisition finding before issuing export permits and re-export certificates. The Parties have agreed that a legal acquisition finding must also be made before issuing certain CITES exemption documents.
(b) Types of legal acquisition. Legal acquisition refers to whether the specimen and its parental stock were:
(1) Obtained in accordance with the provisions of national laws for the protection of wildlife and plants. In the United States, these laws include all applicable local, State, Federal, tribal, and foreign laws; and
(2) If previously traded, traded internationally in accordance with the provisions of CITES.
(c) How we make our findings. We make a finding that a specimen was legally acquired in the following way:
(1) The applicant must provide sufficient information (see § 23.34) for us to make a legal acquisition finding.
(2) We make this finding after considering all available information.
(3) The amount of information we need to make the finding is based on our review of general factors described in paragraph (d) of this section and additional specific factors described in paragraphs (e) through (k) of this section.
(4) As necessary, we consult with foreign Management and Scientific Authorities, the CITES Secretariat, State conservation agencies, Tribes, FWS Law Enforcement, APHIS or CBP, and other appropriate experts.
(d) Risk assessment. We review the general factors listed in this paragraph and additional specific factors in paragraphs (e) through (k) of this section to assess the level of scrutiny and amount of information we need to make a finding of legal acquisition. We give less scrutiny and require less-detailed information when there is a low risk that specimens to be exported or re-exported were not legally acquired, and give more scrutiny and require more detailed information when the proposed activity poses greater risk. We consider the cumulative risks, recognizing that each aspect of the international trade has a continuum of risk from high to low associated with it as follows:
(1) Status of the species: From Appendix I to Appendix III.
(2) Origin of the specimen: From wild-collected to born or propagated in a controlled environment to bred in captivity or artificially propagated.
(3) Source of the propagule used to grow the plant: From documentation that the plant was grown from a non-exempt seed or seedling to documentation that the plant was grown from an exempt seed or seedling.
(4) Origin of the species: From species native to the United States or its bordering countries of Mexico or Canada to nonnative species from other countries.
(5) Volume of illegal trade: From high to low occurrence of illegal trade.
(6) Type of trade: From commercial to noncommercial.
(7) Trade by range countries: From range countries that do not allow commercial export, or allow only limited noncommercial export of the species, to range countries that allow commercial export in high volumes.
(8) Occurrence of the species in a controlled environment in the United States: From uncommon to common in a controlled environment in the United States.
(9) Ability of the species to be bred or propagated readily in a controlled environment: From no documentation that the species can be bred or propagated readily in a controlled environment to widely accepted information that the species is commonly bred or propagated.
(10) Genetic status of the specimen: From a purebred species to a hybrid.
(e) Captive-bred wildlife or a cultivated plant. For a specimen that is captive-bred or cultivated, we may consider whether the parental stock was legally acquired.
(f) Confiscated specimen. For a confiscated Appendix-II or -III specimen, we consider whether information shows that the transfer of the confiscated specimen or its offspring met the conditions of the remission decision, legal settlement, or disposal action after forfeiture or abandonment.
(g) Donated specimen of unknown origin. For an unsolicited specimen of unknown origin donated to a public institution (see § 10.12 of this subchapter), we consider whether:
(1) The public institution follows standard recordkeeping practices and has made reasonable efforts to obtain supporting information on the origin of the specimen.
(2) The public institution provides sufficient information to show it made a reasonable effort to find a suitable recipient in the United States.
(3) The export will provide a conservation benefit to the species.
(4) No persuasive information exists on illegal transactions involving the specimen.
(5) The export is noncommercial, with no money or barter exchanged except for shipping costs.
(6) The institution has no history of receiving a series of rare and valuable specimens or a large quantity of wildlife or plants of unknown origin.
(h) Imported previously. For a specimen that was previously imported into the United States, we consider any reliable, relevant information we receive concerning the validity of a CITES document, regardless of whether the shipment was cleared by FWS, APHIS, or CBP.
(i) Personal use. For a wildlife or plant specimen that is being exported or re-exported for personal use by the applicant, we consider whether:
(1) The specimen was acquired in the United States and possessed for strictly personal use.
(2) The number of specimens is reasonably appropriate for the nature of your export or re-export as personal use.
(3) No persuasive evidence exists on illegal transactions involving the specimen.
(j) Sequential ownership. For a specimen that was previously possessed by someone other than the applicant, we may consider the history of ownership for a specimen and its parental stock, breeding stock, or cultivated parental stock.
(k) Wild-collected in the United States. For a specimen collected from the wild in the United States, we consider the site where the specimen was collected, whether the species is known to occur at that site, the abundance of the species at that site, and, if necessary, whether permission of the appropriate management agency or landowner was obtained to collect the specimen.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.61 What factors are considered in making a non-detriment finding?
(a) Purpose. Articles III and IV of the Treaty require that, before we issue a CITES document, we find that a proposed export or introduction from the sea of Appendix-I or -II specimens is not detrimental to the survival of the species and that a proposed import of an Appendix-I specimen is for purposes that would not be detrimental to the survival of the species.
(b) Types of detriment. Detrimental activities, depending on the species, could include, among other things, unsustainable use and any activities that would pose a net harm to the status of the species in the wild. For Appendix-I species, it also includes use or removal from the wild that results in habitat loss or destruction, interference with recovery efforts for a species, or stimulation of further trade.
(c) General factors. The applicant must provide sufficient information for us to make a finding of non-detriment. In addition to factors in paragraphs (d) and (e) of this section, we will consider whether:
(1) Biological and management information demonstrates that the proposed activity represents sustainable use.
(2) The removal of the animal or plant from the wild is part of a biologically based sustainable-use management plan that is designed to eliminate over-utilization of the species.
(3) If no sustainable-use management plan has been established, the removal of the animal or plant from the wild would not contribute to the over-utilization of the species, considering both domestic and international uses.
(4) The proposed activity, including the methods used to acquire the specimen, would pose no net harm to the status of the species in the wild.
(5) The proposed activity would not lead to long-term declines that would place the viability of the affected population in question.
(6) The proposed activity would not lead to significant habitat or range loss or restriction.
(d) Additional factor for Appendix-II species. In addition to the general factors in paragraph (c) of this section, we will consider whether the intended export of an Appendix-II species would cause a significant risk that the species would qualify for inclusion in Appendix I.
(e) Additional factors for Appendix-I species. In addition to the general factors in paragraph (c) of this section, we will consider whether the proposed activity:
(1) Would not cause an increased risk of extinction for either the species as a whole or the population from which the specimen was obtained.
(2) Would not interfere with the recovery of the species.
(3) Would not stimulate additional trade in the species. If the proposed activity does stimulate trade, we will consider whether the anticipated increase in trade would lead to the decline of the species.
(f) How we make our findings. We base the non-detriment finding on the best available biological information. We also consider trade information, including trade demand, and other scientific management information. We make a non-detriment finding in the following way:
(1) We consult with the States, Tribes, other Federal agencies, scientists, other experts, and the range countries of the species.
(2) We consult with the Secretariat and other Parties to monitor the level of trade that is occurring in the species.
(3) Based on the factors in paragraphs (c) through (e) of this section, we evaluate the biological impact of the proposed activity.
(4) In cases where insufficient information is available or the factors above are not satisfactorily addressed, we take precautionary measures and would be unable to make the required finding of non-detriment.
(g) Risk assessment. We review the status of the species in the wild and the degree of risk the proposed activity poses to the species to determine the level of scrutiny needed to make a finding. We give greater scrutiny and require more detailed information for activities that pose a greater risk to a species in the wild. We consider the cumulative risks, recognizing that each aspect of international trade has a continuum of risk (from high to low) associated with it as follows:
(1) Status of the species: From Appendix I to Appendix II.
(2) Origin of the specimen: From wild-collected to born or propagated in a controlled environment to bred in captivity or artificially propagated.
(3) Source of the propagule used to grow the plant: From documentation that the plant was grown from a non-exempt seed or seedling to documentation that the plant was grown from an exempt seed or seedling.
(4) Origin of the species: From native species to nonnative species.
(5) Volume of legal trade: From high to low occurrence of legal trade.
(6) Volume of illegal trade: From high to low occurrence of illegal trade.
(7) Type of trade: From commercial to noncommercial.
(8) Genetic status of the specimen: From a purebred species to a hybrid.
(9) Risk of disease transmission: From high to limited risk of disease transmission.
(10) Basis for listing: From listed under Article II(1) or II(2)(a) of the Treaty to listed under Article II(2)(b).
(h) Quotas for Appendix-I species. When an export quota has been set by the CoP for an Appendix-I species, we will consider the scientific and management basis of the quota together with the best available biological information when we make our non-detriment finding. We will contact the Scientific and Management Authorities of the exporting country for further information if needed.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.62 What factors are considered in making a finding of not for primarily commercial purposes?
(a) Purpose. Under Article III(3(c)) and (5(c)) of the Treaty, an import permit or an introduction-from-the-sea certificate for Appendix-I species can be issued only if the Management Authority is satisfied that the specimen is not to be used for primarily commercial purposes. Trade in Appendix-I species must be subject to particularly strict regulation and authorized only in exceptional circumstances.
(b) How we make our findings. We must find that the intended use of the Appendix-I specimen is not for primarily commercial purposes before we can issue a CITES document.
(1) We will make this decision on a case-by-case basis considering all available information.
(2) The applicant must provide sufficient information to satisfy us that the intended use is not for primarily commercial purposes.
(3) The definitions of “commercial” and “primarily commercial purposes” in § 23.5 apply.
(4) We will look at all aspects of the intended use of the specimen. If the noncommercial aspects do not clearly predominate, we will consider the import or introduction from the sea to be for primarily commercial purposes.
(5) While the nature of the transaction between the owner in the country of export and the recipient in the country of import or introduction from the sea may have some commercial aspects, such as the exchange of money to cover the costs of shipment and care of specimens during transport, it is the intended use of the specimen, including the purpose of the export, that must not be for primarily commercial purposes.
(6) We will conduct an assessment of factors listed in paragraph (d) of this section. For activities involving an anticipated measurable increase in revenue and other economic value associated with the intended use, we will conduct an analysis as described in paragraph (e) of this section.
(7) All net profits generated in the United States from activities associated with the import of an Appendix-I species must be used for conservation of that species.
(c) Examples. The following are examples of types of transactions in which the noncommercial aspects of the intended use of the specimen may predominate depending on the facts of each situation. The discussions of each example provide further guidance in assessing the actual degree of commerciality on a case-by-case basis. These examples outline circumstances commonly encountered and do not cover all situations where import or introduction from the sea could be found to be not for primarily commercial purposes.
(1) Personal use. Import or introduction from the sea of an Appendix-I specimen for personal use generally is considered to be not for primarily commercial purposes. An example is the import of a personal sport-hunted trophy by the person who hunted the wildlife for display in his or her own home.
(2) Scientific purposes. The import or introduction from the sea of an Appendix-I specimen by a scientist or scientific institution may be permitted in situations where resale, commercial exchange, or exhibit of the specimen for economic benefit is not the primary intended use.
(3) Conservation, education, or training. Generally an Appendix-I specimen may be imported or introduced from the sea by government agencies or nonprofit institutions for purposes of conservation, education, or training. For example, a specimen could be imported or introduced from the sea primarily to train customs staff in effective CITES control, such as for identification of certain types of specimens.
(4) Biomedical industry. Import or introduction from the sea of an Appendix-I specimen by an institution or company in the biomedical industry is initially presumed to be commercial since specimens are typically imported or introduced from the sea to develop and sell products that promote public health for profit. However, if the importer clearly shows that the sale of products is only incidental to public health research and not for the primary purpose of economic benefit or profit, then such an import or introduction from the sea could be considered as scientific research under paragraph (c)(2) of this section if the principles of paragraph (b) of this section are met.
(5) Captive-breeding or artificial propagation programs. The import of an Appendix-I specimen for purposes of establishing a commercial operation for breeding or artificial propagation is considered to be for primarily commercial purposes. As a general rule, import or introduction from the sea of an Appendix-I specimen for a captive-breeding or artificial propagation program must have as a priority the long-term protection and recovery of the species in the wild. The captive-breeding or artificial propagation program must be part of a program aimed at the recovery of the species in the wild and be undertaken with the support of a country within the species' native range. Any profit gained must be used to support this recovery program. If a captive-breeding or artificial propagation operation plans to sell surplus specimens to help offset the costs of its program, import or introduction from the sea would be allowed only if any profit would be used to support the captive-breeding or artificial propagation program to the benefit of the Appendix-I species, not for the personal economic benefit of a private individual or share-holder.
(6) Professional dealers. Import or introduction from the sea by a professional dealer who states a general intention to eventually sell the specimen or its offspring to an undetermined recipient would be considered to be for primarily commercial purposes. However, import or introduction from the sea through a professional dealer by a qualified applicant may be acceptable if the ultimate intended use would be for one of the purposes set out in paragraphs (c)(2), (3), and (5) of this section and where a binding contract, conditioned on the issuing of permits, is in place.
(d) Risk assessment. We review the factors listed in this paragraph (d) to assess the level of scrutiny and amount of information we need to make a finding of whether the intended use of the specimen is not for primarily commercial purposes. We give less scrutiny and require less detailed information when the import or introduction from the sea poses a low risk of being primarily commercial, and give more scrutiny and require more detailed information when the proposed activity poses greater risk. We consider the cumulative risks, recognizing that each aspect of the international trade has a continuum of risk from high to low associated with it as follows:
(1) Type of importer: From for-profit entity to private individual to nonprofit entity.
(2) Ability of the proposed uses to generate revenue: From the ability to generate measurable increases in revenue or other economic value to no anticipated increases in revenue or other economic value.
(3) Appeal of the species: From high public appeal to low public appeal.
(4) Occurrence of the species in the United States: From uncommon to common in a controlled environment in the United States.
(5) Intended use of offspring: From commercial to noncommercial.
(e) Analysis of anticipated revenues and other economic value. We will analyze revenues and other economic value anticipated to result from the use of the specimen for activities with a high risk of being primarily commercial.
(1) We will examine the proposed use of any net profits generated in the United States. We consider net profit to include all funds or other valuable considerations (including enhanced value of common stock shares) received or attained by you or those affiliated with you as a result of the import or introduction from the sea, to the extent that such funds or other valuable considerations exceed the reasonable expenses that are properly attributable to the proposed activity.
(2) We will consider any conservation project to be funded and, if the species was or is to be taken from the wild, how the project benefits the species in its native range, including agreements, timeframes for accomplishing tasks, and anticipated benefits to the species.
(3) We will consider any plans to monitor a proposed conservation project, including expenditure of funds or completion of tasks.
(4) In rare cases involving unusually high net profits, we will require the applicant to provide a detailed analysis of expected revenue (both direct and indirect) and expenses to show anticipated net profit, and a statement from a licensed, independent certified public accountant that the internal accounting system is sufficient to account for and track funds generated by the proposed activities.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.63 What factors are considered in making a finding that an animal is bred in captivity?
(a) Purpose. Article VII(4) and (5) of the Treaty provide exemptions that allow for the special treatment of wildlife that was bred in captivity (see §§ 23.41 and 23.46).
(b) Definitions. The following terms apply when determining whether specimens qualify as “bred in captivity”:
(1) A controlled environment means one that is actively manipulated for the purpose of producing specimens of a particular species; that has boundaries designed to prevent specimens, including eggs or gametes, from entering or leaving the controlled environment; and has general characteristics that may include artificial housing, waste removal, provision of veterinary care, protection from predators, and artificially supplied food.
(2) Breeding stock means an ensemble of captive wildlife used for reproduction.
(c) Bred-in-captivity criteria. For a specimen to qualify as bred in captivity, we must be satisfied that all the following criteria are met:
(1) If reproduction is sexual, the specimen was born to parents that either mated or transferred gametes in a controlled environment.
(2) If reproduction is asexual, the parent was in a controlled environment when development of the offspring began.
(3) The breeding stock meets all of the following criteria:
(i) Was established in accordance with the provisions of CITES and relevant national laws.
(ii) Was established in a manner not detrimental to the survival of the species in the wild.
(iii) Is maintained with only occasional introduction of wild specimens as provided in paragraph (d) of this section.
(iv) Has consistently produced offspring of second or subsequent generations in a controlled environment, or is managed in a way that has been demonstrated to be capable of reliably producing second-generation offspring and has produced first-generation offspring.
(d) Addition of wild specimens. A very limited number of wild specimens (including eggs or gametes) may be introduced into a breeding stock if all of the following conditions are met (for Appendix-I specimens see also § 23.46(b)(12)):
(1) The specimens were acquired in accordance with the provisions of CITES and relevant national laws.
(2) The specimens were acquired in a manner not detrimental to the survival of the species in the wild.
(3) The specimens were added either to prevent or alleviate deleterious inbreeding, with the number of specimens added as determined by the need for new genetic material, or to dispose of confiscated animals.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.64 What factors are considered in making a finding that a plant is artificially propagated?
(a) Purpose. Article VII(4) and (5) of the Treaty provide exemptions that allow for special treatment of plants that were artificially propagated (see §§ 23.40 and 23.47).
(b) Definitions. The following terms apply when determining whether specimens qualify as “artificially propagated”:
(1) Controlled conditions means a nonnatural environment that is intensively manipulated by human intervention for the purpose of plant production. General characteristics of controlled conditions may include, but are not limited to, tillage, fertilization, weed and pest control, irrigation, or nursery operations such as potting, bedding, or protection from weather.
(2) Cultivated parental stock means the ensemble of plants grown under controlled conditions that are used for reproduction.
(c) Artificially propagated criteria. Except as provided in paragraphs (f) and (g) of this section, for a plant specimen to qualify as artificially propagated, we must be satisfied that the plant specimen was grown under controlled conditions from a seed, cutting, division, callus tissue, other plant tissue, spore, or other propagule that either is exempt from the provisions of CITES or has been derived from cultivated parental stock. The cultivated parental stock must meet all of the following criteria:
(1) Was established in accordance with the provisions of CITES and relevant national laws.
(2) Was established in a manner not detrimental to the survival of the species in the wild.
(3) Is maintained in sufficient quantities for propagation so as to minimize or eliminate the need for augmentation from the wild, with such augmentation occurring only as an exception and limited to the amount necessary to maintain the vigor and productivity of the cultivated parental stock.
(d) Cutting or division. A plant grown from a cutting or division is considered to be artificially propagated only if the traded specimen does not contain any material collected from the wild.
(e) Grafted plant. A grafted plant is artificially propagated only when both the rootstock and the material grafted to it have been taken from specimens that were artificially propagated in accordance with paragraph (c) of this section. A grafted specimen that consists of taxa from different Appendices is treated as a specimen of the taxon listed in the more restrictive Appendix.
(f) Timber. Timber taken from trees planted and grown in a monospecific plantation is considered artificially propagated if the seeds or other propagules from which the trees are grown were legally acquired and obtained in a non-detrimental manner.
(g) Exception for certain plant specimens grown from wild-collected seeds or spores. Plant specimens grown from wild-collected seeds or spores may be considered artificially propagated only when all of the following conditions have been met:
(1) Establishment of a cultivated parental stock for the taxon presents significant difficulties because specimens take a long time to reach reproductive age.
(2) The seeds or spores are collected from the wild and grown under controlled conditions within a range country, which must also be the country of origin of the seeds or spores.
(3) The Management Authority of the range country has determined that the collection of seeds or spores was legal and consistent with relevant national laws for the protection and conservation of the species.
(4) The Scientific Authority of the range country has determined that collection of the seeds or spores was not detrimental to the survival of the species in the wild, and allowing trade in such specimens has a positive effect on the conservation of wild populations. In making these determinations, all of the following conditions must be met:
(i) The collection of seeds or spores for this purpose must be limited in such a manner as to allow regeneration of the wild population.
(ii) A portion of the plants produced must be used to establish plantations to serve as cultivated parental stock in the future and become an additional source of seeds or spores and thus reduce or eliminate the need to collect seeds or spores from the wild.
(iii) A portion of the plants produced must be used for replanting in the wild, to enhance recovery of existing populations or to re-establish populations that have been extirpated.
(5) Operations propagating Appendix–I species for commercial purposes must be registered with the CITES Secretariat in accordance with the Guidelines for the registration of nurseries exporting artificially propagated specimens of Appendix–I species.
Credits
[79 FR 30427, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.65 What factors are considered in making a finding that an applicant is suitably equipped to house and care for a live specimen?
(a) Purpose. Under Article III(3)(b) and (5)(b) of the Treaty, an import permit or introduction-from-the-sea certificate for live Appendix-I specimens can be issued only if we are satisfied that the recipients are suitably equipped to house and care for them.
(b) General principles. We will follow these general principles in making a decision on whether an applicant has facilities that would provide proper housing to maintain the specimens for the intended purpose and the expertise to provide proper care and husbandry or horticultural practices.
(1) All persons who would be receiving a specimen must be identified in an application and their facilities approved by us, including persons who are likely to receive a specimen within 1 year after it arrives in the United States.
(2) The applicant must provide sufficient information for us to make a finding, including, but not limited to, a description of the facility, photographs, or construction plans, and resumes of the recipient or staff who will care for the specimen.
(3) We use the best available information on the requirements of the species in making a decision and will consult with experts and other Federal and State agencies, as necessary and appropriate.
(4) The degree of scrutiny that we give an application is based on the biological and husbandry or horticultural needs of the species.
(c) Specific factors considered for wildlife. In addition to the general provisions in paragraph (e) of this section, we consider the following factors in evaluating suitable housing and care for wildlife:
(1) Enclosures constructed and maintained so as to provide sufficient space to allow each animal to make normal postural and social adjustments with adequate freedom of movement. Inadequate space may be indicated by evidence of malnutrition, poor condition, debility, stress, or abnormal behavior patterns.
(2) Appropriate forms of environmental enrichment, such as nesting material, perches, climbing apparatus, ground substrate, or other species-specific materials or objects.
(3) If the wildlife is on public display, an off-exhibit area, consisting of indoor and outdoor accommodations, as appropriate, that can house the wildlife on a long-term basis if necessary.
(4) Provision of water and nutritious food of a nature and in a way that are appropriate for the species.
(5) Staff who are trained and experienced in providing proper daily care and maintenance for the species being imported or introduced from the sea, or for a closely related species.
(6) Readily available veterinary care or veterinary staff experienced with the species or a closely related species, including emergency care.
(d) Specific factors considered for plants. In addition to the general provisions in paragraph (e) of the section, we consider the following factors in evaluating suitable housing and care for plants:
(1) Sufficient space, appropriate lighting, and other environmental conditions that will ensure proper growth.
(2) Ability to provide appropriate culture, such as water, fertilizer, and pest and disease control.
(3) Staff with experience with the imported species or related species with similar horticultural requirements.
(e) General factors considered for wildlife and plants. In addition to the specific provisions in paragraphs (c) or (d) of this section, we will consider the following factors in evaluating suitable housing and care for wildlife and plants:
(1) Adequate enclosures or holding areas to prevent escape or unplanned exchange of genetic material with specimens of the same or different species outside the facility.
(2) Appropriate security to prevent theft of specimens and measures taken to rectify any previous theft or security problem.
(3) A reasonable survival rate of specimens of the same species or, alternatively, closely related species at the facility, mortalities for the previous 3 years, significant injuries to wildlife or damage to plants, occurrence of significant disease outbreaks during the previous 3 years, and measures taken to prevent similar mortalities, injuries, damage, or diseases. Significant injuries, damage, or disease outbreaks are those that are permanently debilitating or re-occurring.
(4) Sufficient funding on a long-term basis to cover the cost of maintaining the facility and the specimens imported.
(f) Incomplete facilities or insufficient staff. For applications submitted to us before the facilities to hold the specimen are completed or the staff is identified or properly trained, we will:
(1) Review all available information, including construction plans or intended staffing, and make a finding based on this information.
(2) Place a condition on any permit that the import cannot occur until the facility has been completed or the staff hired and trained, and approved by us.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
Subpart E. International Trade in Certain Specimens
§ 23.68 How can I trade internationally in roots of American ginseng?
(a) U.S. and foreign general provisions. Whole plants and roots (whole, sliced, and parts, excluding manufactured parts, products, and derivatives, such as powders, pills, extracts, tonics, teas, and confectionery) of American ginseng (Panax quinquefolius), whether wild or artificially propagated, are included in Appendix II. Cultivated American ginseng that does not meet the requirements of artificially propagated will be considered wild for export and re-export purposes. The import, export, or re-export of ginseng roots must meet the requirements of this section and other requirements of this part (see subparts B and C for prohibitions and application procedures). For specimens that were harvested from a State or Tribe without an approved CITES export program, see § 23.36 for export permits and § 23.37 for re-export certificates.
(b) Export approval of State and tribal programs. States and Tribes set up and maintain ginseng management and harvest programs designed to monitor and protect American ginseng from over-harvest. When a State or Tribe with a management program provides us with the necessary information, we make programmatic findings and have specific requirements that allow export under CITES. For wild ginseng, a State or Tribe must provide sufficient information for us to determine that its management program and harvest controls are appropriate to ensure that ginseng harvested within its jurisdiction is legally acquired and that export will not be detrimental to the survival of the species in the wild. For artificially propagated ginseng, a State or Tribe must provide sufficient information for us to determine that ginseng grown within its jurisdiction meets the definition of artificially propagated and the State or Tribe must have procedures in place to minimize the risk that the roots of wild-collected plants would be claimed as artificially propagated.
(1) A State or Tribe seeking initial CITES export program approval for wild or artificially propagated American ginseng must submit the following information on the adoption and implementation of regulatory measures to the U.S. Management Authority:
(i) Laws or regulations mandating licensing or registration of persons buying and selling ginseng in that State or on tribal lands.
(ii) A requirement that ginseng dealers maintain records and provide copies of those records to the appropriate State or tribal management agency upon request. Dealer records must contain: the name and address of the ginseng seller, date of transaction, whether the ginseng is wild or artificially propagated and dried or green at time of transaction, weight of roots, State or Tribe of origin of roots, and identification numbers of the State or tribal certificates used to ship ginseng from the State or Tribe of origin.
(iii) A requirement that State or tribal personnel will inspect roots, ensure legal harvest, and have the ability to determine the age of roots of all wild-collected ginseng harvested in the State or on tribal lands. State or tribal personnel may accept a declaration statement by the licensed or registered dealer or grower that the ginseng roots are artificially propagated.
(iv) A requirement that State or tribal personnel will weigh ginseng roots unsold by March 31 of the year after harvest and give a weight receipt to the owner of the roots. Future export certification of this stock must be issued against the weight receipt.
(v) A requirement that State or tribal personnel will issue certificates for wild and artificially propagated ginseng. These certificates must contain at a minimum:
(A) State of origin.
(B) Serial number of certificate.
(C) Dealer's State or tribal license or registration number.
(D) Dealer's shipment number for that harvest season.
(E) Year of harvest of ginseng being certified.
(F) Designation as wild or artificially propagated.
(G) Designation as dried or fresh (green) roots.
(H) Weight of roots.
(I) Statement of State or tribal certifying official verifying that the ginseng was obtained in that State or on those tribal lands in accordance with all relevant laws for that harvest year.
(J) Name and title of State or tribal certifying official.
(2) In addition, a State or Tribe seeking initial CITES export program approval for wild American ginseng must submit the following information to the U.S. Management Authority:
(i) An assessment of the condition of the population and trends, including a description of the types of information on which the assessment is based, such as an analysis of population demographics; population models; or analysis of past harvest levels or indices of abundance independent of harvest information, such as field surveys.
(ii) Historic, present, and potential distribution of wild ginseng on a county-by-county basis.
(iii) Phenology of ginseng, including flowering and fruiting periods.
(iv) Habitat evaluation.
(v) If available, copies of any ginseng management or monitoring plans or other relevant reports that the State or Tribe has prepared as part of its existing management program.
(3) A State or Tribe with an approved CITES export program must complete Form 3-200-61 and submit it to the U.S. Management Authority by May 31 of each year to provide information on the previous harvest season.
(c) U.S. application process. Application forms and a list of States and Tribes with approved ginseng programs can be obtained from our website or by contacting us (see § 23.7).
(1) To export wild or artificially propagated ginseng harvested under an approved State or tribal program, complete Form 3-200-34 or Form 3-200-74 for additional single-use permits under an annual program file.
(2) To export wild ginseng harvested from a State or Tribe that does not have an approved program, complete Form 3-200-32. To export artificially propagated ginseng from a State or Tribe that does not have an approved program, complete Form 3-200-33.
(3) To re-export ginseng, complete Form 3-200-32.
(4) For information on issuance criteria for CITES documents, see § 23.36 for export permits, § 23.37 for re-export certificates, and § 23.40 for certificates for artificially propagated plants.
(d) Conditions for export. Upon export, roots must be accompanied by a State or tribal certificate containing the information specified in paragraph (b)(1)(v) of this section.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.69 How can I trade internationally in fur skins and fur skin products of bobcat, river otter, Canada lynx, gray wolf, and brown bear?
(a) U.S. and foreign general provisions. For purposes of this section, CITES furbearers means bobcat (Lynx rufus), river otter (Lontra canadensis), Canada lynx (Lynx canadensis), gray wolf (Canis lupus), and brown bear (Ursus arctos) harvested in the United States. These species are included in Appendix II based on Article II(2)(b) of the Treaty (see § 23.89). The import, export, or re-export of fur skins and fur skin products must meet the requirements of this section and the other requirements of this part (see subparts B and C for prohibitions and application procedures). For specimens that were harvested from a State or Tribe without an approved CITES export program, see § 23.36 for export permits and § 23.37 for re-export certificates.
(b) Export approval of State and tribal programs. States and Tribes set up and maintain management and harvest programs designed to monitor and protect CITES furbearers from over-harvest. When a State or Tribe with a management program provides us with the necessary information, we make programmatic findings and have specific requirements that allow export under CITES. A State or Tribe must provide sufficient information for us to determine that its management program and harvest controls are appropriate to ensure that CITES furbearers harvested within its jurisdiction are legally acquired and that export will not be detrimental to the survival of the species in the wild.
(1) A State or Tribe seeking initial CITES export program approval must submit the following information to the U.S. Management Authority, except as provided in paragraph (b)(2) of this section:
(i) An assessment of the condition of the population and a description of the types of information on which the assessment is based, such as an analysis of carcass demographics, population models, analysis of past harvest levels as a function of fur prices or trapper effort, or indices of abundance independent of harvest information, such as scent station surveys, archer surveys, camera traps, track or scat surveys, or road kill counts.
(ii) Current harvest control measures, including laws regulating harvest seasons and methods.
(iii) Total allowable harvest of the species.
(iv) Distribution of harvest.
(v) Indication of how frequently harvest levels are evaluated.
(vi) Tagging or marking requirements for fur skins.
(vii) Habitat evaluation.
(viii) If available, copies of any furbearer management plans or other relevant reports that the State or Tribe has prepared as part of its existing management program.
(2) If the U.S. Scientific Authority has made a range-wide non-detriment finding for a species, a State or Tribe seeking initial approval for a CITES export program for that species need only submit the information in (b)(1)(ii) and (vi) of this section.
(3) A State or Tribe with an approved CITES export program must submit a CITES furbearer activity report to the U.S. Management Authority by October 31 of each year that provides information as to whether or not the population status or management of the species has changed within the State or tribal lands. This report may reference information provided in previous years if the information has not changed. Except as provided in paragraph (b)(4) of this section, a furbearer activity report should include, at a minimum, the following:
(i) For each species, the number of specimens taken and the number of animals tagged, if different.
(ii) An assessment of the condition of the population, including trends, and a description of the types of information on which the assessment is based. If population levels are decreasing, the activity report should include the State or Tribe's professional assessment of the reason for the decline and any steps being taken to address it.
(iii) Information on, and a copy of, any changes in laws or regulations affecting these species.
(iv) If available, copies of relevant reports that the State or Tribe has prepared during the year in question as part of its existing management programs for CITES furbearers.
(4) When the U.S. Scientific Authority has made a range-wide non-detriment finding for a species, the annual furbearer activity report from a State or Tribe with an approved export program for that species should include, at a minimum, a statement indicating whether or not the status of the species has changed and the information in paragraph (b)(3)(iii) and (iv) of this section. Range-wide non-detriment findings will be re-evaluated at least every 5 years, or sooner if information indicates that there has been a change in the status or management of the species that might lead to different treatment of the species. When a range-wide non-detriment finding is re-evaluated, States and Tribes with an approved export program for the species must submit information that allows us to determine whether our finding remains valid.
(c) CITES tags. Unless an alternative method has been approved, each CITES fur skin to be exported or re-exported must have a U.S. CITES tag permanently attached.
(1) The tag must be inserted through the skin and permanently locked in place using the locking mechanism of the tag.
(2) The legend on the CITES tag must include the US–CITES logo, an abbreviation for the State or Tribe of harvest, a standard species code assigned by the Management Authority, and a unique serial number.
(3) Fur skins without a CITES tag permanently attached may not be exported or re-exported. If the CITES tag has been inadvertently removed, damaged, or lost you may obtain a replacement tag. To obtain a replacement tag, either from the State or Tribe that issued the original tag or from us, you must provide information to show that the fur was legally acquired.
(i) When a tag is inadvertently removed, damaged, or lost, you may contact the State or Tribe of harvest for a replacement tag. If the State or Tribe cannot replace it, you may apply to FWS Law Enforcement for a replacement tag. If the tag has been inadvertently removed or damaged, you must give us the tag. If the tag is lost, you must provide details concerning how the tag was lost. If we are satisfied that the fur was legally acquired, we will provide a CITES replacement tag.
(ii) A replacement tag must meet all of the requirements in paragraph (c) of this section, except the legend will include only the US–CITES logo, FWS–REPL, and a unique serial number.
(4) Tags are not required on fur skin products.
(d) Documentation requirements. The U.S. CITES export permit or an annex attached to the permit must contain all information that is given on the tag.
(e) U.S. application process. Application forms and a list of States and Tribes with approved furbearer programs can be obtained from our website or by contacting us (see § 23.7).
(1) To export fur skins taken under an approved State or tribal program, complete Form 3–200–26 and submit it to either FWS Law Enforcement or the U.S. Management Authority.
(2) To export fur skins that were not harvested under an approved program or to export products made from fur skins, complete Form 3–200–27 and submit it to the U.S. Management Authority.
(3) To re-export fur skins or products made from fur skins, complete Form 3–200–73 and submit it either to FWS Law Enforcement or the U.S. Management Authority.
(4) For information on issuance criteria for CITES documents, see § 23.36 for export permits and § 23.37 for re-export certificates.
(f) Conditions for export. Upon export, each fur skin, other than a fur skin product, must be clearly identified in accordance with paragraph (c) of this section.
Credits
[79 FR 30427, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.70 How can I trade internationally in American alligator and other crocodilian skins, parts, and products?
(a) U.S. and foreign general provisions. For the purposes of this section, crocodilian means all species of alligator, caiman, crocodile, and gavial of the order Crocodylia. The import, export, or re-export of any crocodilian skins, parts, or products must meet the requirements of this section and the other requirements of this part (see subparts B and C for prohibitions and application procedures). For American alligator (Alligator mississippiensis) specimens harvested from a State or Tribe without an approved CITES export program, see § 23.36 for export permits and § 23.37 for re-export certificates.
(b) Definitions. Terms used in this section are defined as follows:
(1) Crocodilian skins means whole or partial skins, flanks, chalecos, and bellies (including those that are salted, crusted, tanned, partially tanned, or otherwise processed), including skins of sport-hunted trophies.
(2) Crocodilian parts means body parts with or without skin attached (including tails, throats, feet, meat, skulls, and other parts) and small cut skin pieces.
(c) Export approval of State and tribal programs for American alligator. States and Tribes set up and maintain management and harvest programs designed to monitor and protect American alligators from over-harvest. When a State or Tribe with a management program provides us with the necessary information, we make programmatic findings and have specific requirements that allow export under CITES. A State or Tribe must provide sufficient information for us to determine that its management program and harvest controls are appropriate to ensure that alligators harvested within its jurisdiction are legally acquired and that the export will not be detrimental to the survival of the species in the wild.
(1) A State or Tribe seeking initial CITES export program approval must submit the following to the U.S. Management Authority:
(i) An assessment of the condition of the wild population and a description of the types of information on which the assessment is based, such as an analysis of carcass demographics, population models, analysis of past harvest levels as a function of skin prices or harvester effort, or indices of abundance independent of harvest information, such as nest surveys, spotlighting surveys, or nuisance complaints.
(ii) Current harvest control measures, including laws regulating harvest seasons and methods.
(iii) Total allowable harvest of the species.
(iv) Distribution of harvest.
(v) Indication of how frequently harvest levels are evaluated.
(vi) Tagging or marking requirements for skins and parts.
(vii) Habitat evaluation.
(viii) Information on nuisance alligator management programs.
(ix) Information on alligator farming programs, including whether collecting and rearing of eggs or hatchlings is allowed, what factors are used to set harvest levels, and whether any alligators are returned to the wild.
(x) If available, copies of any alligator management plans or other relevant reports for American alligator that the State or Tribe has prepared as part of its existing management program.
(2) A State or Tribe with an approved CITES export program must submit an American alligator activity report to the U.S. Management Authority by July 1 of each year to provide information regarding harvests during the previous year. This report may reference information provided in previous years if the information has not changed. An American alligator activity report, at a minimum, should include the following:
(i) The total number of skins from wild or farmed alligators that were tagged by the State or Tribe.
(ii) An assessment of the status of the alligator population with an indication of whether the population is stable, increasing, or decreasing, and at what rate (if known). If population levels are decreasing, activity reports should include the State or Tribe's professional assessment of the reason for the decline and any steps being taken to address it.
(iii) For wild alligators, information on harvest, including harvest of nuisance alligators, methods used to determine harvest levels, demographics of the harvest, and methods used to determine the total number and population trends of alligators in the wild.
(iv) For farmed alligators, information on whether collecting and rearing of eggs or hatchlings is allowed, what factors are used to set harvest levels, and whether any alligators are returned to the wild.
(v) Information on, and a copy of, any changes in laws or regulations affecting the American alligator.
(vi) If available, copies of relevant reports that the State or Tribe has prepared during the reporting period as part of its existing management program for the American alligator.
(3) We provide CITES export tags to States and Tribes with approved CITES export programs. American alligator skins and parts must meet the marking and tagging requirements of paragraphs (d), (e), and (f) of this section.
(d) Tagging of crocodilian skins. You may import, export, or re-export any crocodilian skin only if a non-reusable tag is inserted though the skin and locked in place using the locking mechanism of the tag. A mounted sport-hunted trophy must be accompanied by the tag from the skin used to make the mount.
(1) Except as provided for a replacement tag in paragraph (d)(3)(ii) of this section, the tag must:
(i) Be tamper-resistant, self-locking, heat resistant, and inert to chemical and mechanical processes.
(ii) Be permanently stamped with the two-letter ISO code for the country of origin, a unique serial number, a standardized species code (available on our Web site; see § 23.7), and for specimens of species from populations that have been transferred from Appendix I to Appendix II for ranching, the year of skin production or harvest. For American alligator, the export tags include the US–CITES logo, an abbreviation for the State or Tribe of harvest, a standard species code (MIS = Alligator mississippiensis), the year of skin production or harvest, and a unique serial number.
(iii) If the year of skin production or harvest and serial number appear next to each other on a tag, the information should be separated by a hyphen.
(2) Skins, flanks, and chalecos must be individually tagged.
(3) Skins without a non-reusable tag permanently attached may not be exported or re-exported. To obtain a replacement tag, either from the State or Tribe of harvest (for American alligator) or from us, you must provide information to show that the skin was legally acquired.
(i) In the United States, when an American alligator tag is inadvertently removed, damaged, or lost, you may contact the State or Tribe of harvest for a replacement tag. If the State or Tribe cannot replace it, you may apply to FWS Law Enforcement for a replacement tag. To obtain replacement tags for crocodilian skins other than American alligator in the United States, contact FWS Law Enforcement. If the tag has been inadvertently removed or damaged, you must give us the tag. If the tag is lost, you must provide details concerning how the tag was lost. If we are satisfied that the skin was legally acquired, we will provide a CITES replacement tag.
(ii) A replacement tag must meet all of the requirements in paragraph (d)(1) of this section except that the species code and year of skin production or harvest will not be required, and for re-exports the country of re-export must be shown in place of the country of origin. In the United States, the legend will include the US–CITES logo, FWS–REPL, and a unique serial number.
(e) Meat and skulls. Except for American alligator, you may import, export, or re-export crocodilian meat and skulls without tags or markings. American alligator meat and skulls may be imported, exported, or re-exported if packaged and marked or tagged in accordance with State or tribal laws as follows:
(1) Meat from legally harvested and tagged alligators must be packed in permanently sealed containers and labeled as required by State or tribal laws or regulations. Bulk meat containers must be marked with any required State or tribal parts tag or bulk meat tag permanently attached and indicating, at a minimum, State or Tribe of origin, year of take, species, original U.S. CITES tag number for the corresponding skin, weight of meat in the container, and identification of State-licensed processor or packer.
(2) Each American alligator skull must be marked as required by State or tribal law or regulation.
(f) Tagging or labeling of crocodilian parts other than meat and skulls. You may import, export, or re-export crocodilian parts other than meat and skulls when the following conditions are met:
(1) Parts must be packed in transparent sealed containers.
(2) Containers must be clearly marked with a non-reusable parts tag or label that includes all of the information in paragraph (d)(1)(ii) of this section and a description of the contents, the total weight (contents and container), and the number of the CITES document.
(3) Tags are not required on crocodilian products.
(4) Tags are not required on scientific specimens except as required in paragraphs (d) and (e) of this section.
(g) Documentation requirements. The CITES document or an annex attached to the document must contain all information that is given on the tag or label.
(h) U.S. application process. Application forms and a list of States and Tribes with approved American alligator programs can be obtained from our website or by contacting us (see § 23.7).
(1) To export American alligator specimens taken under an approved State or tribal program, except for products made from American alligators, complete Form 3–200–26 and submit it to either FWS Law Enforcement or the U.S. Management Authority.
(2) To export American alligator specimens that are not from an approved program, or to export products made from American alligators, complete Form 3–200–27 and submit it to the U.S. Management Authority.
(3) To re-export crocodilian specimens, complete Form 3–200–73 and submit it to either FWS Law Enforcement or the U.S. Management Authority.
(4) For information on issuance criteria for CITES documents, see § 23.36 for export permits and § 23.37 for re-export certificates.
(i) Conditions for import, export, or re-export. Upon import, export, or re-export, each crocodilian specimen must meet the applicable tagging requirements in paragraphs (d), (e), and (f) of this section.
Credits
[79 FR 30427, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.71 How can I trade internationally in sturgeon caviar?
(a) U.S. and foreign provisions. For the purposes of this section, sturgeon caviar or caviar means the processed roe of any species of sturgeon or paddlefish (order Acipenseriformes). It does not include sturgeon or paddlefish eggs contained in shampoos, cosmetics, lotions, or other products for topical application. The import, export, or re-export of sturgeon caviar must meet the requirements of this section and the other requirements of this part. The import, export, or re-export of Acipenseriformes specimens other than caviar must meet the other requirements of this part. See subparts B and C for prohibitions and application procedures.
(b) Labeling. You may import, export, or re-export sturgeon caviar only if labels are affixed to containers prior to export or re-export in accordance with this paragraph.
(1) The following definitions apply to caviar labeling:
(i) Non-reusable label means any label or mark that cannot be removed without being damaged or transferred to another container. In the United States, the design of the label will be determined by the labeler in accordance with the requirements of this section.
(ii) Primary container means any container (tin, jar, pail or other receptacle) in direct contact with the caviar.
(iii) Secondary container means the receptacle into which primary containers are placed.
(iv) Processing plant means a facility in the country of origin responsible for the first packaging of caviar into a primary container. In the United States, this may be done by the person who harvested the roe.
(v) Repackaging plant means a facility responsible for receiving and repackaging caviar into new primary containers. This includes any facility where caviar is removed from the container in which it was received and placed in a different container.
(vi) Lot identification number means a number that corresponds to information related to the caviar tracking system used by the processing plant or repackaging plant.
(2) The caviar-processing plant in the country of origin must affix a non-reusable label on the primary container that includes all of the following information:
(i) Standardized species code; for hybrids, the species code for the male is followed by the code for the female and the codes are separated by an “x” (codes are available on our website; see § 23.7).
(ii) Source code.
(iii) Two-letter ISO code of the country of origin.
(iv) Year of harvest. This is either the calendar year in which caviar was harvested or, for caviar imported from shared stocks subject to quotas, the quota year in which it was harvested.
(v) Processing plant code and lot identification number.
(3) If caviar is repackaged before export or re-export, the repackaging plant must affix a non-reusable label to the primary container that includes all of the following information:
(i) The standardized species code, source code, and two-letter ISO code of the country of origin.
(ii) Year of repackaging and the repackaging plant code, which incorporates the two-letter ISO code for the repackaging country if different from the country of origin.
(iii) Lot identification number or, for caviar that is being re-exported, the CITES document number under which it was imported may be used in place of the lot identification number.
(4) The exact quantity of caviar must be indicated on any secondary container along with a description of the contents in accordance with international customs regulations.
(c) Documentation requirements. Unless the sturgeon caviar qualifies as a personal or household effect under § 23.15, the CITES document or an annex attached to the document must contain all information that is given on the label. The exact quantity of each species of caviar must be indicated on the CITES document.
(d) Export quotas. Commercial shipments of sturgeon caviar from stocks shared between different countries may be imported only if all of the following conditions have been met:
(1) The relevant countries have established annual export quotas for the shared stocks that were derived from catch quotas agreed among the countries. The quotas are based on an appropriate regional conservation strategy and monitoring regime and are not detrimental to the survival of the species in the wild.
(2) The quotas have been communicated to the CITES Secretariat and the Secretariat has communicated the annual export quotas to CITES Parties.
(3) The caviar is exported during the quota year (March 1 - last day of February) in which it was harvested and processed.
(4) [Reserved by 73 FR 40986]
(e) Re-exports. Any re-export of sturgeon caviar must occur within 18 months from the date of issuance of the original export permit.
(f) Pre–Convention. Sturgeon caviar may not be imported, exported, or re-exported under a pre–Convention certificate.
(g) Mixed caviar. Caviar that consists of roe from more than one species may only be imported into or exported from the United States if the exact quantity of roe from each species is known and is indicated on the CITES document.
(h) U.S. application forms. Application forms can be obtained from our website or by contacting us (see § 23.7). For CITES document requirements, see § 23.36 for export permits and § 23.37 for re-export certificates. For export, complete Form 3–200–76 or Form 3–200–80 and submit it to the U.S. Management Authority. For re-export, complete Form 3–200–73 and submit it either to FWS Law Enforcement or the U.S. Management Authority.
(i) CITES register of exporters and of processing and repackaging plants. The CITES Secretariat maintains a “Register of licensed exporters and of processing and repackaging plants for specimens of sturgeon and paddlefish species” on its Web site. If you hold a current import-export license issued by FWS Law Enforcement and wish to be added to the CITES register, you may submit your contact information and processing or repackaging plant codes to the U.S. Management Authority for submission to the CITES Secretariat.
Credits
[73 FR 40986, July 17, 2008; 79 FR 30428, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.72 How can I trade internationally in plants?
(a) U.S. and foreign general provisions: In addition to the requirements of this section, the import, export, or re-export of CITES plant specimens must meet the other requirements of this part (see subparts B and C for prohibitions and application procedures).
(b) Seeds. International shipments of seeds of any species listed in Appendix I, except for seeds of certain artificially propagated hybrids (see § 23.92), or seeds of species listed in Appendix II or III with an annotation that includes seeds, must be accompanied by a valid CITES document. International shipments of CITES seeds that are artificially propagated also must be accompanied by a valid CITES document.
(c) A plant propagated from exempt plant material. A plant grown from exempt plant material is regulated by CITES.
(1) The proposed shipment of the specimen is treated as an export even if the exempt plant material from which it was derived was previously imported. The country of origin is the country in which the specimen ceased to qualify for the exemption.
(2) Plants grown from exempt plant material qualify as artificially propagated provided they are grown under controlled conditions.
(3) To export plants grown from exempt plant material under controlled conditions, complete Form 3-200-33 for a certificate for artificially propagated plants.
(d) Salvaged plants.
(1) For purposes of this section, salvaged plant means a plant taken from the wild as a result of some environmental modification in a country where a Party has done all of the following:
(i) Ensured that the environmental modification program does not threaten the survival of CITES plant species, and that protection of Appendix-I species in situ is considered a national and international obligation.
(ii) Established salvaged specimens in cultivation after concerted attempts have failed to ensure that the environmental modification program would not put at risk wild populations of CITES species.
(2) International trade in salvaged Appendix-I plants, and Appendix-II plants whose entry into trade might otherwise have been considered detrimental to the survival of the species in the wild, may be permitted only when all the following conditions are met:
(i) Such trade would clearly benefit the survival of the species in the wild or in cultivation.
(ii) Import is for the purposes of care and propagation.
(iii) Import is by a bona fide botanic garden or scientific institution.
(iv) Any salvaged Appendix-I plant will not be sold or used to establish a commercial operation for artificial propagation after import.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.73 How can I trade internationally in timber?
(a) U.S. and foreign general provisions: In addition to the requirements of this section, the import, export, or re-export of timber species listed under CITES must meet the other requirements of this part (see subparts B and C for prohibitions and application procedures).
(b) Definitions. The following definitions apply to parts, products, and derivatives that appear in the annotations to certain timber species in the CITES Appendices. These definitions are based on the tariff classifications of the Harmonized System of the World Customs Organization.
(1) Logs means all wood in the rough, whether or not stripped of bark or sapwood, or roughly squared for processing, notably into sawn wood, pulpwood, or veneer sheets.
(2) Sawn wood means wood simply sawn lengthwise or produced by a profile-chipping process. Sawn wood normally exceeds 6 mm in thickness.
(3) Veneer sheets means thin layers or sheets of wood of uniform thickness, usually 6 mm or less, usually peeled or sliced, for use in making plywood, veneer furniture, veneer containers, or similar products.
(4) Plywood means wood material consisting of three or more sheets of wood glued and pressed one on the other and generally disposed so that the grains of successive layers are at an angle.
(c) The following exceptions apply to Appendix-II or -III timber species that have a substantive annotation that designates either logs, sawn wood, and veneer sheets, or logs, sawn wood, veneer sheets, and plywood:
(1) Change in destination. When a shipment of timber destined for one country is redirected to another, the Management Authority in the country of import may change the name and address of the importer indicated on the CITES document under the following conditions:
(i) The quantity imported is the same as the quantity certified by a stamp or seal and authorized signature of the Management Authority on the CITES document at the time of export or re-export.
(ii) The number of the bill of lading for the shipment is on the CITES document, and the bill of lading is presented at the time of import.
(iii) The import takes place before the CITES document expires, and the period of validity has not been extended.
(iv) The Management Authority of the importing country includes the following statement in block 5, or an equivalent place, of the CITES document: “Import into [name of country] permitted in accordance with [cite the appropriate section number from the current permit and certificate resolution] on [date].” The modification is certified with an official stamp and signature.
(v) The Management Authority sends a copy of the amended CITES document to the country of export or re-export and the Secretariat.
(2) Extension of CITES document validity. A Management Authority in the country of import may extend the validity of an export permit or re-export certificate beyond the normal maximum of 6 months after the date of issue under the following conditions:
(i) The shipment has arrived in the port of final destination before the CITES document expires, is being held in customs bond, and is not considered imported.
(ii) The time extension does not exceed 6 months from the date of expiration of the CITES document and no previous extension has been issued.
(iii) The Management Authority has included in block 5, or an equivalent place, of the CITES document the date of arrival and the new date of expiration on the document, and certified the modification with an official stamp and signature.
(iv) The shipment is imported into the country from the port where the Management Authority issued the extension and before the amended CITES document expires.
(v) The Management Authority sends a copy of the amended CITES document to the country of export or re-export and to the Secretariat.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.74 How can I trade internationally in personal sport-hunted trophies?
(a) U.S. and foreign general provisions. Except as provided for personal and household effects in § 23.15, the import, export, or re-export of sport-hunted trophies of species listed under CITES must meet the requirements of this section and the other requirements of this part (see subparts B and C for prohibitions and application procedures).
(b) Sport-hunted trophy means a whole dead animal or a readily recognizable part or derivative of an animal specifically identified on accompanying CITES documents that meets the following criteria:
(1) Is raw, processed, or manufactured;
(2) Was legally obtained by the hunter through hunting for his or her personal use;
(3) Is being imported, exported, or re-exported by or on behalf of the hunter as part of the transfer from its country of origin ultimately to the hunter's country of usual residence; and
(4) Includes worked, manufactured, or handicraft items made from the sport-hunted animal only when:
(i) Such items are contained in the same shipment as raw or tanned parts of the sport-hunted animal and are for the personal use of the hunter;
(ii) The quantity of such items is no more than could reasonably be expected given the number of animals taken by the hunter as shown on the license or other documentation of the authorized hunt accompanying the shipment; and
(iii) The accompanying CITES documents (export document and, if appropriate, import permit) contain a complete itemization and description of all items included in the shipment.
(c) Use after import. You may use your sport-hunted trophy after import into the United States as provided in § 23.55.
(d) Quantity. The following provisions apply to the issuance and acceptance of U.S. and foreign documents for sport-hunted trophies originating from a population for which the Conference of the Parties has established an export quota. The number of trophies that one hunter may import in any calendar year for the following species is:
(1) No more than two leopard (Panthera pardus) trophies.
(2) No more than one markhor (Capra falconeri) trophy.
(3) No more than one black rhinoceros (Diceros bicornis) trophy.
(e) Marking or tagging.
(1) The following provisions apply to the issuance and acceptance of U.S. and foreign documents for sport-hunted trophies originating from a population for which the Conference of the Parties has established an export quota. Each trophy imported, exported, or re-exported must be marked or tagged in the following manner:
(i) Leopard and markhor: Each raw or tanned skin must have a self-locking tag inserted through the skin and permanently locked in place using the locking mechanism of the tag. The tag must indicate the country of origin, the number of the specimen in relation to the annual quota, and the calendar year in which the specimen was taken in the wild. A mounted sport-hunted trophy must be accompanied by the tag from the skin used to make the mount.
(ii) Black rhinoceros: Parts of the trophy, including, but not limited to, skin, skull, or horns, whether mounted or loose, should be individually marked with reference to the country of origin, species, the number of the specimen in relation to the annual quota, and the year of export.
(iii) Crocodilians: See marking requirements in § 23.70.
(iv) The export permit or re-export certificate or an annex attached to the permit or certificate must contain all the information that is given on the tag.
(2) African elephant (Loxodonta africana). The following provisions apply to the issuance and acceptance of U.S. and foreign documents for sport-hunted trophies of African elephant. The trophy ivory must be legibly marked by means of punch-dies, indelible ink, or other form of permanent marking, under a marking and registration system established by the country of origin, with the following formula: The country of origin represented by the corresponding two-letter ISO country code; the last two digits of the year in which the elephant was harvested for export; the serial number for the year in question; and the weight of the ivory in kilograms. The mark must be highlighted with a flash of color and placed on the lip mark area. The lip mark area is the area of a whole African elephant tusk where the tusk emerges from the skull and which is usually denoted by a prominent ring of staining on the tusk in its natural state.
Credits
[79 FR 30428, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.75 How can I trade internationally in vicuña (Vicugna vicugna)?
(a) U.S. and foreign general provisions. The import, export, or re-export of specimens of vicuña must meet the requirements of this section and the other requirements of this part (see subparts B and C of this part for prohibitions and application procedures). Certain populations of vicuña are listed in Appendix II for the exclusive purpose of allowing international trade in wool sheared from live vicuñas, cloth made from such wool, and products manufactured from such wool or cloth. All other specimens of vicuña are deemed to be specimens of a species included in Appendix I.
(b) Vicuña Convention means the Convenio para la Conservación y Manejo de la Vicuña, of which vicuña range countries are signatories.
(c) Vicuña logotype means the logotype adopted by the vicuña range countries under the Vicuña Convention.
(d) Country of origin for the purposes of the vicuña label means the name of the country where the vicuña wool in the cloth or product originated.
(e) Wool sheared from live vicuñas, cloth from such wool, and products manufactured from such wool or cloth may be imported from Appendix–II populations only when they meet the labeling requirements in paragraph (f) of this section.
(f) Labeling requirements. Except for cloth containing CITES pre–Convention wool of vicuña, you may import, export, or re-export vicuña cloth only when the reverse side of the cloth bears the vicuña logotype and the selvages bear the words “VICUÑA--COUNTRY OF ORIGIN”. Specimens of other products manufactured from vicuña wool or cloth must bear a label that has the vicuña logotype and the designation “VICUÑA--COUNTRY OF ORIGIN--ARTESANIA”. Each specimen must bear such a label. For import into the United States of raw wool sheared from live vicuña, see the labeling requirements in 50 CFR 17.40(m).
Credits
[79 FR 30429, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
Subpart F. Disposal of Confiscated Wildlife and Plants
§ 23.78 What happens to confiscated wildlife and plants?
(a) Purpose. Article VIII of the Treaty provides for confiscation or return to the country of export of specimens that are traded in violation of CITES.
(b) Disposal options. Part 12 of this subchapter provides the options we have for disposing of forfeited and abandoned live and dead wildlife and plants. These include maintenance in captivity either in the United States or in the country of export, return to the wild under limited circumstances, and sale of certain Appendix-II or -III specimens. Under some conditions, euthanasia or destruction may be necessary.
(1) We use a plant rescue center program to dispose of confiscated live plants. Participants in this program may also assist APHIS, CBP, and FWS Law Enforcement in holding seized specimens as evidence pending any legal decisions.
(2) We dispose of confiscated live wildlife on a case-by-case basis at the time of seizure and forfeiture, and consider the quantity, protection level, and husbandry needs of the wildlife.
(c) Re-export. We may issue a re-export certificate for a CITES specimen that was forfeited or abandoned when the certificate indicates the specimen was confiscated and when the re-export meets one of the following purposes:
(1) For any CITES species, the return of a live specimen to the Management Authority of the country of export, placement of a live specimen in a rescue center, or use of the specimen for law enforcement, judicial, or forensic purposes.
(2) For an Appendix-II or -III species, the disposal of the specimen in an appropriate manner that benefits enforcement and administration of the Convention.
(d) Consultation process. FWS and APHIS may consult with the Management Authority in the country of export or re-export and other relevant governmental and nongovernmental experts before making a decision on the disposal of confiscated live specimens that have been forfeited or abandoned to the FWS, APHIS, or CBP.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
23.79 How may I participate in the Plant Rescue Center Program?
(a) Purpose. We have established the Plant Rescue Center Program to place confiscated live plants quickly to prevent physical damage to the plants.
(b) Criteria. Institutions interested in participating in this program must be:
(1) Nonprofit, open to the public, and have the expertise and facilities to care for confiscated exotic plant specimens. A participating institution may be a botanical garden, arboretum, zoological park, research institution, or other qualifying institution.
(2) Willing to transfer confiscated plants from the port where they were confiscated to their facilities at their own expense.
(3) Willing to return the plants to the U.S. Government if the country of export has requested their return. The U.S. Government will then coordinate the plants' return to the country of export.
(4) Willing to accept and maintain a plant shipment as a unit until it has received authorization from us to incorporate the shipment into its permanent collection or transfer a portion of it to another participating institution.
(c) Participation. Institutions wishing to participate in the Plant Rescue Center Program should contact the U.S. Management Authority (see § 23.7). They must provide a brief description of the greenhouse or display facilities, the names and telephone numbers of any individuals authorized to accept plants on behalf of the institution, and the mailing address where the plants should be sent. In addition, interested institutions must indicate if they are limited with regard to the type of plants they are able to maintain or the quantities of plants they can handle at one time.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
Subpart G. Cites Administration
§ 23.84 What are the roles of the Secretariat and the committees?
(a) Secretariat. The Secretariat is headed by the Secretary–General. Its functions are listed in Article XII of the Treaty and include:
(1) Arranging and staffing meetings of the Parties.
(2) Performing functions as requested in relation to listings in the Appendices.
(3) Undertaking scientific and technical studies, as authorized by the CoP, to contribute to implementation of the Convention.
(4) Studying reports of the Parties and requesting additional information as appropriate to ensure effective implementation of the Convention.
(5) Bringing to the attention of the Parties matters relevant to the Convention.
(6) Periodically publishing and distributing to the Parties current editions of the Appendices as well as information on the identification of specimens of species listed in the Appendices.
(7) Preparing annual reports to the Parties on its work and on the implementation of the Convention.
(8) Making recommendations for the implementation of the aims and provisions of the Convention, including the exchange of scientific and technical information.
(9) Performing other functions entrusted to it by the Parties.
(b) Committees. The Parties have established three committees to provide administrative and technical support to the Parties and to the Secretariat. The CoP may charge any of these committees with tasks.
(1) The Standing Committee steers the work and performance of the Convention between CoPs.
(i) This committee oversees development and execution of the Secretariat's budget, advises other committees, appoints working groups, and carries out activities on behalf of the Parties between CoPs.
(ii) Regional representatives are countries that are elected by their respective geographic regions at the CoP.
(2) The Animals Committee and the Plants Committee provide advice and guidance to the CoP, the other committees, working groups, and the Secretariat on all matters relevant to international trade in species included in the Appendices.
(i) These committees also develop and maintain a standardized list of species names; provide assistance with regard to identification of species listed in the Appendices; cooperate with the Secretariat to assist Scientific Authorities; compile and evaluate data on Appendix–II species that are considered significantly affected by trade; periodically review the status of wildlife and plant species listed in the Appendices; advise range countries on management techniques when requested; draft resolutions on wildlife and plant matters for consideration by the Parties; deal with issues related to the transport of live specimens; and report to the CoP and the Standing Committee.
(ii) Regional representatives are individuals, who are elected by their respective geographic regions at the CoP.
(iii) The CoP appoints a specialist in zoological nomenclature to the Animals Committee and a specialist in botanical nomenclature to the Plants Committee. These specialists are ex officio and non-voting, and are responsible for developing or identifying standard nomenclature references for wildlife and plant taxa and making recommendations on nomenclature to Parties, the CoP, other committees, working groups, and the Secretariat.
Credits
[79 FR 30429, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.85 What is a meeting of the Conference of the Parties (CoP)?
(a) Purpose. Article XI of the Treaty provides general guidelines for meetings of the countries that have ratified, accepted, approved, or acceded to CITES. The Parties currently meet for 2 weeks every 3 years. At these meetings, the Parties consider amendments to the Appendices and resolutions and decisions to improve the implementation of CITES. The Parties adopt amendments to the lists of species in Appendix I and II and resolutions by a two-thirds majority of Parties present and voting. The Secretariat or any Party may also submit reports on wildlife and plant trade for consideration.
(b) CoP locations and dates. At a CoP, Parties interested in hosting the next meeting notify the Secretariat. The Parties vote to select the location of the next CoP. Once a country has been chosen, it works with the Secretariat to set the date and specific venue. The Secretariat then notifies the Parties of the date for the next CoP.
(c) Attendance at a CoP. All Parties may participate and vote at a CoP. Non-Party countries may participate, but may not vote. Organizations technically qualified in protection, conservation, or management of wildlife or plants may participate in a CoP as observers if they are approved, but they are not eligible to vote.
(1) International organizations must apply to the CITES Secretariat for approval to attend a CoP as an observer.
(2) National organizations must apply to the Management Authority of the country where they are located for approval to attend a CoP as an observer.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.86 How can I obtain information on a CoP?
As we receive information on an upcoming CoP from the CITES Secretariat, we will notify the public either through published notices in the Federal Register or postings on our website (see § 23.7). We will provide:
(a) A summary of the information we have received with an invitation for the public to comment and provide information on the agenda, proposed amendments to the Appendices, and proposed resolutions that they believe the United States should submit for consideration at the CoP.
(b) Information on times, dates, and locations of public meetings.
(c) Information on how international and national organizations may apply to participate as observers.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.87 How does the United States develop documents and negotiating positions for a CoP?
(a) In developing documents and negotiating positions for a CoP, we:
(1) Will provide for at least one public meeting.
(2) Consult with appropriate Federal, State, and tribal agencies; foreign governmental agencies; scientists; experts; and others.
(3) Seek public comment through published Federal Register notices or postings on our website that:
(i) Solicit recommendations on potential proposals to amend the Appendices, draft resolutions, and other documents for U.S. submission to the CoP.
(ii) Announce proposals to amend the Appendices, draft resolutions, and other documents that the United States is considering submitting to the CoP.
(iii) Provide the CoP agenda and a list of the amendments to the Appendices proposed for the CoP, a summary of our proposed negotiating positions on these items, and the reasons for our proposed positions.
(4) Consider comments received in response to notices or postings provided in paragraph (a)(3) of this section.
(b) We submit the following documents to the Secretariat for consideration at the CoP:
(1) Draft resolutions and other documents at least 150 days before the CoP.
(2) Proposals to amend the Appendices at least 150 days before the CoP if we have consulted all range countries, or 330 days before the CoP if we have not consulted the range countries. For the latter, the additional time allows for the range countries to be consulted through the Secretariat.
(c) The Director may modify or suspend any of these procedures if they would interfere with the timely or appropriate development of documents for submission to the CoP and U.S. negotiating positions.
(d) We may receive additional information at a CoP or circumstances may develop that have an impact on our tentative negotiating positions. As a result, the U.S. representatives to a CoP may find it necessary to modify, reverse, or otherwise change any of those positions when to do so would be in the best interests of the United States or the conservation of the species.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.88 What are the resolutions and decisions of the CoP?
(a) Purpose. Under Article XI of the Treaty, the Parties agree to resolutions and decisions that clarify and interpret the Convention to improve its effectiveness. Resolutions are generally intended to provide long-standing guidance, whereas decisions typically contain instructions to a specific committee, Parties, or the Secretariat. Decisions are often intended to be implemented by a specific date, and then they expire.
(b) Effective date. A resolution or decision adopted by the Parties becomes effective 90 days after the last day of the meeting at which it was adopted, unless otherwise specified in the resolution or decision.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
Subpart H. Lists of Species
§ 23.89 What are the criteria for listing species in Appendix I or II?
(a) Purpose. Article XV of the Treaty sets out the procedures for amending CITES Appendices I and II. A species must meet trade and biological criteria listed in the CITES resolution for amendment of Appendices I and II. When determining whether a species qualifies for inclusion in or removal from Appendix I or II, or transfer from one Appendix to another, we will:
(1) Consult with States, Tribes, range countries, relevant experts, other Federal agencies, and the general public.
(2) Utilize the best available biological information.
(3) Evaluate that information against the criteria in paragraphs (b) through (f) of this section.
(b) Listing a species in Appendix I. Any species qualifies for inclusion in Appendix I if it is or may be affected by trade and meets, or is likely to meet, at least one biological criterion for Appendix I.
(1) These criteria are:
(i) The size of the wild population is small.
(ii) Area of distribution is restricted.
(iii) There is an observed, inferred, or projected marked decline in the population size in the wild.
(2) Factors to be considered include, but are not limited to, population and range fragmentation; habitat availability or quality; area of distribution; taxon-specific vulnerabilities due to life history, behavior, or other intrinsic factors, such as migration; population structure and niche requirements; threats from extrinsic factors such as the form of exploitation, introduced species, habitat degradation and destruction, and stochastic events; or decreases in recruitment.
(c) Listing a species in Appendix II due to actual or potential threats. Any species qualifies for inclusion in Appendix II if it is or may be affected by trade and meets at least one of the criteria for listing in Appendix II based on actual or potential threats to that species. These criteria are:
(1) It is known, or can be inferred or projected, that the regulation of trade is necessary to avoid the species becoming eligible for inclusion in Appendix I in the near future.
(2) It is known, or can be inferred or projected, that the regulation of trade in the species is required to ensure that the harvest of specimens from the wild is not reducing the wild population to a level at which its survival might be threatened by continued harvest or other influences.
(d) Listing a species in Appendix II due to similarity of appearance or other factors. Any species qualifies for inclusion in Appendix II if it meets either of the criteria for listing in Appendix II due to similarity of appearance or other factors. These criteria are:
(1) The specimens of the species in the form in which they are traded resemble specimens of a species listed in Appendix II due to criteria in paragraph (c) of this section or in Appendix I, such that enforcement officers who encounter specimens of such similar CITES species are unlikely to be able to distinguish between them.
(2) There are compelling reasons other than those in paragraph (d)(1) of this section to ensure that effective control of trade in currently listed species is achieved.
(e) Other issues. We will evaluate any potential changes to the Appendices, taking into consideration other issues, including but not limited to, split-listing, annotation, listings of higher taxa and hybrids, and specific listing issues related to plants and commercially exploited aquatic species.
(f) Precautionary measures. We will evaluate any potential transfers from Appendix I to II or removal of species from the Appendices in the context of precautionary measures.
(g) Proposal. If a Party determines that a taxon qualifies for inclusion in or removal from Appendix I or II, or transfer from one Appendix to another, a proposal may be submitted to the Secretariat for consideration by the CoP.
(1) The proposal should indicate the intent of the specific action (such as inclusion in Appendix I or II); be specific and accurate as to the parts and derivatives to be included in the listing; ensure that any proposed annotation is consistent with existing annotations; state the criteria against which the proposal is to be judged; and provide a justification for the basis on which the species meets the relevant criteria.
(2) The proposal must be in a prescribed format. Contact the U.S. Scientific Authority for a copy (see § 23.7).
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.90 What are the criteria for listing species in Appendix III?
(a) Purpose. Article XVI of the Treaty sets out the procedures for amending Appendix III.
(b) General procedure. A Party may unilaterally, at any time, submit a request to list a species in Appendix III to the CITES Secretariat. The listing will become effective 90 days after the Secretariat notifies the Parties of the request.
(c) Criteria for listing. For a Party to list a species in Appendix III, all of the following criteria must be met:
(1) The species must be native to the country listing the species.
(2) The species must be protected under that country's laws or regulations to prevent or restrict exploitation and control trade, and the laws or regulations are being implemented.
(3) The species is in international trade, and there are indications that the cooperation of other Parties would help to control illegal trade.
(4) The listing Party must inform the Management Authorities of other range countries, the known major importing countries, the Secretariat, and the Animals Committee or the Plants Committee that it is considering the listing and seek their opinions on the potential effects of the listing.
(d) Annotation. The listing Party may annotate the Appendix-III listing to include only specific parts, products, derivatives, or life stages, as long as the Secretariat is notified of the annotation.
(e) U.S. procedure. The procedure to list a species native to the United States in Appendix III is as follows:
(1) We will consult with and solicit comments from all States and Tribes where the species occurs and all other range countries.
(2) We will publish a proposed rule in the Federal Register to solicit comments from the public.
(3) If after evaluating the comments received and available information we determine the species should be listed in Appendix III, we will publish a final rule in the Federal Register and notify the Secretariat of the listing.
(f) Removing a species from Appendix III. We will monitor the international trade in Appendix-III species listed by us and periodically evaluate whether each species continues to meet the listing criteria in paragraph (c) of this section. We will remove a species from Appendix III provided all of the following criteria are met:
(1) International trade in the species is very limited. As a general guide, we will consider removal when exports involve fewer than 5 shipments per year or fewer than 100 individual animals or plants.
(2) Legal and illegal trade in the species, including international trade or interstate commerce, is determined not to be a concern.
(g) Transferring a species from Appendix III to Appendix I or II. If, after monitoring the trade and evaluating the status of an Appendix-III species we listed, we determine that the species meets the criteria in § 23.89(b) through (d) of this section for listing in Appendix I or II, we will consider whether to submit a proposal to amend the listing at the next CoP.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.91 How do I find out if a species is listed?
(a) CITES list. The official CITES list includes species of wildlife and plants placed in Appendix I, II, and III in accordance with the provisions of Articles XV and XVI of the Treaty. This list is maintained by the CITES Secretariat based on decisions of the Parties. You may access the official list from the CITES website (see § 23.7).
(b) Effective date. Amendments to the CITES list are effective as follows:
(1) Appendix-I and -II species listings adopted at the CoP are effective 90 days after the last day of the CoP, unless otherwise specified in the proposal.
(2) Appendix-I and -II species listings adopted between CoPs by postal procedures are effective 120 days after the Secretariat has communicated comments and recommendations on the listing to the Parties if the Secretariat does not receive an objection to the proposed amendment from a Party.
(3) Appendix-III species listings are effective 90 days after the date the Secretariat has communicated such listings to the Parties. A listing Party may withdraw a species from the list at any time by notifying the Secretariat. The withdrawal is effective 30 days after the Secretariat has communicated the withdrawal to the Parties.
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.
§ 23.92 Are any wildlife or plants, and their parts, products, or derivatives, exempt?
(a) All living or dead wildlife and plants in Appendix I, II, and III and all their readily recognizable parts, products, and derivatives must meet the requirements of CITES and this part, except as indicated in paragraphs (b) and (c) of this section.
(b) The following are exempt from the requirements of CITES. You may be required to demonstrate that your specimen qualifies as exempt under this section. For specimens that are exempt from CITES requirements, you must still follow the clearance requirements for wildlife in part 14 of this subchapter and for plants in part 24 of this subchapter and 7 CFR parts 319, 352, and 355.
(1) Appendix–III wildlife and Appendix–II or -III plants.
(i) Where an annotation designates what is excluded from CITES requirements, any part, product, or derivative that is specifically excluded.
(ii) Where an annotation designates what is covered by the Treaty, all parts, products, or derivatives that are not designated.
(2) Plant hybrids. Specimens of an Appendix–II or -III plant taxon with an annotation that specifically excludes hybrids.
(c) The following are exempt from CITES document requirements when certain criteria are met.
(1) Plant hybrids. Seeds and pollen (including pollinia), cut flowers, and flasked seedlings or tissue cultures of hybrids that qualify as artificially propagated (see § 23.64) and that were produced from one or more Appendix–I species or taxa that are not annotated to treat hybrids as Appendix–I specimens.
(2) Flasked seedlings of Appendix–I orchids. Flasked seedlings of an Appendix–I orchid species that qualify as artificially propagated (see § 23.64).
(3) Marine specimens listed in Appendix II that are protected under another treaty, convention, or international agreement which was in force on July 1, 1975 as provided in § 23.39(d).
(4) Coral sand and coral fragments as defined in § 23.5.
(5) Personal and household effects as provided in § 23.15.
(6) Urine, feces, and synthetically derived DNA as provided in § 23.16.
(7) Certain wildlife hybrids as provided in § 23.43.
Credits
[79 FR 30429, May 27, 2014]
SOURCE: 72 FR 48448, Aug. 23, 2007, unless otherwise noted.
AUTHORITY: Convention on International Trade in Endangered Species of Wild Fauna and Flora (March 3, 1973), 27 U.S.T. 1087; and Endangered Species Act of 1973, as amended, 16 U.S.C. 1531 et seq.